
MIRI Coronagraphy Pipeline Notebook#
Authors: B. Nickson; MIRI branch
Last Updated: November 10, 2025
Pipeline Version: 1.20.2 (Build 12.1)
Purpose:
This notebook provides a framework for processing generic Mid-Infrared Instrument (MIRI) Coronagraphic data through all three James Webb Space Telescope (JWST) pipeline stages. Data is assumed to be located in separate observation folders according to the paths set up below. Editing cells other than those in the Configuration should not be necessary unless the standard pipeline processing options are modified.
Data:
This example is set up to use F1550C coronagraphic observations of the super-Jupiter exoplanet HIP 65426 b, obtained by Program ID 1386 (PI: S. Hinkley). It incorporates observations of the exoplanet host star HIP 65426 at two separate roll angles (1 exposure each); a PSF reference observation of the nearby star HIP 65219, taken with a 9-pt small grid dither pattern (9 exposures total); a background observation associated with the target star, taken with a 2-pt dither (two exposures); and a background observation associated with the PSF reference target, taken with a 2-pt dither (two exposures).
The relevant observation numbers are:
Science observations: 8, 9
Science backgrounds: 30
Reference observations: 7
Reference backgrounds: 31
Example input data to use will be downloaded automatically unless disabled (i.e., to use local files instead).
JWST pipeline version and CRDS context:
This notebook was written for the above-specified pipeline version and associated build context for this version of the JWST Calibration Pipeline. Information about this and other contexts can be found in the JWST Calibration Reference Data System (CRDS server). If you use different pipeline versions, please refer to the table here to determine what context to use. To learn more about the differences for the pipeline, read the relevant documentation.
Please note that pipeline software development is a continuous process, so results in some cases may be slightly different if a subsequent version is used. For optimal results, users are strongly encouraged to reprocess their data using the most recent pipeline version and associated CRDS context, taking advantage of bug fixes and algorithm improvements. Any known issues for this build are noted in the notebook.
Updates:
This notebook is regularly updated as improvements are made to the pipeline. Find the most up to date version of this notebook at:
spacetelescope/jwst-pipeline-notebooks
Recent Changes:
Jan 28, 2025: Migrate from the Coronagraphy_ExambleNB notebook, update to Build 11.2 (jwst 1.17.1).
May 5, 2025: Updated to jwst 1.18.0 (no significant changes)
July 16, 2025: Updated to jwst 1.19.1 (no significant changes)
November 10, 2025: Updated to jwst 1.20.2 (no significant changes)
Table of Contents#
Configuration
Package Imports
Demo Mode Setup
Directory Setup
Detector1 Pipeline
Image2 Pipeline
Coron3 Pipeline
Plot the spectra
1.-Configuration#
Set basic parameters to use with this notebook. These will affect what data is used, where data is located (if already in disk), and pipeline modules run on this data. The list of parameters are as follows:
demo_mode
directories with data
mask
filter
pipeline modules
# Basic import necessary for configuration
import os
demo_mode must be set appropriately below.
Set demo_mode = True to run in demonstration mode. In this mode, this
notebook will download example data from the
Barbara A. Mikulski Archive for Space Telescopes (MAST) and process it through the pipeline.
This will all happen in a local directory unless modified
in Section 3 below.
Set demo_mode = False if you want to process your own data that has already
been downloaded and provide the location of the data.
# Set parameters for demo_mode, mask, filter, data mode directories, and
# processing steps.
# -------------------------------Demo Mode---------------------------------
demo_mode = True
if demo_mode:
print('Running in demonstration mode using online example data!')
# -------------------------Data Mode Directories---------------------------
# If demo_mode = False, look for user data in these paths
if not demo_mode:
# Set directory paths for processing specific data; these will need
# to be changed to your local directory setup (below are given as
# examples)
basedir = os.path.join(os.path.expanduser('~'), 'FlightData1386/')
# Point to where science observation data are
# Assumes uncalibrated data in sci_r1_dir/uncal/ and sci_r2_dir/uncal/,
# and results in stage1, stage2, stage3 directories
sci_r1_dir = os.path.join(basedir, 'sci_r1/')
sci_r2_dir = os.path.join(basedir, 'sci_r2/')
# Point to where reference target observation data are
# Assumes uncalibrated data in ref_dir/uncal/ and results in stage1,
# stage2, stage3 directories
ref_targ_dir = os.path.join(basedir, 'ref_targ/')
# Point to where background observation data are
# Assumes uncalibrated data in sci_bg_dir/uncal/ and ref_targ_bg_dir/uncal/,
# and results in stage1, stage2 directories
bg_sci_dir = os.path.join(basedir, 'bg_sci/')
bg_ref_targ_dir = os.path.join(basedir, 'bg_ref_targ/')
# --------------------------Set Processing Steps--------------------------
# Whether or not to process only data from a given coronagraphic mask/
# filter (useful if overriding reference files)
# Note that BOTH parameters must be set in order to work
use_mask = '4QPM_1550' # '4QPM_1065', '4QPM_1140', '4QPM_1550', or 'LYOT_2300'
use_filter = 'F1550C' # 'F1065C', 'F1140C', 'F1550C', or 'F2300C'
# Individual pipeline stages can be turned on/off here. Note that a later
# stage won't be able to run unless data products have already been
# produced from the prior stage.
# Science processing
dodet1 = True # calwebb_detector1
doimage2 = True # calwebb_image2
docoron3 = True # calwebb_coron3
# Background processing
dodet1bg = True # calwebb_detector1
doimage2bg = True # calwebb_image2
Running in demonstration mode using online example data!
Set CRDS context and server#
Before importing CRDS and JWST modules, we need to configure our environment. This includes defining a CRDS cache directory in which to keep the reference files that will be used by the calibration pipeline.
If the root directory for the local CRDS cache directory has not been set already, it will be set to create one in the home directory.
# ------------------------Set CRDS context and paths----------------------
# Each version of the calibration pipeline is associated with a specific CRDS
# context file. The pipeline will select the appropriate context file behind
# the scenes while running. However, if you wish to override the default context
# file and run the pipeline with a different context, you can set that using
# the CRDS_CONTEXT environment variable. Here we show how this is done,
# although we leave the line commented out in order to use the default context.
# If you wish to specify a different context, uncomment the line below.
#%env CRDS_CONTEXT jwst_1322.pmap
# Check whether the local CRDS cache directory has been set.
# If not, set it to the user home directory
if (os.getenv('CRDS_PATH') is None):
os.environ['CRDS_PATH'] = os.path.join(os.path.expanduser('~'), 'crds')
# Check whether the CRDS server URL has been set. If not, set it.
if (os.getenv('CRDS_SERVER_URL') is None):
os.environ['CRDS_SERVER_URL'] = 'https://jwst-crds.stsci.edu'
# Echo CRDS path in use
print('CRDS local filepath:', os.environ['CRDS_PATH'])
print('CRDS file server:', os.environ['CRDS_SERVER_URL'])
CRDS local filepath: /home/runner/crds
CRDS file server: https://jwst-crds.stsci.edu
2.-Package Imports#
# Use the entire available screen width for this notebook
from IPython.display import display, HTML
display(HTML("<style>.container { width:95% !important; }</style>"))
# Basic system utilities for interacting with files
# ----------------------General Imports------------------------------------
import glob
#import copy
import time
from pathlib import Path
import re
# Numpy for doing calculations
import numpy as np
# -----------------------Astropy Imports-----------------------------------
# Astropy utilities for opening FITS and ASCII files, and downloading demo files
from astropy.io import fits
from astropy.wcs import WCS
from astropy.coordinates import SkyCoord
#from astropy import time
from astroquery.mast import Observations
# -----------------------Plotting Imports----------------------------------
# Matplotlib for making plots
import matplotlib.pyplot as plt
# --------------JWST Calibration Pipeline Imports---------------------------
# Import the base JWST and calibration reference files packages
import jwst
import crds
# JWST pipelines (each encompassing many steps)
from jwst.pipeline import Detector1Pipeline
from jwst.pipeline import Image2Pipeline
from jwst.pipeline import Coron3Pipeline
# JWST pipeline utilities
from jwst import datamodels # JWST datamodels
from jwst.associations import asn_from_list as afl # Tools for creating association files
from jwst.associations.lib.rules_level2_base import DMSLevel2bBase # Definition of a Lvl2 association file
from jwst.associations.lib.rules_level3_base import DMS_Level3_Base # Definition of a Lvl3 association file
from jwst.stpipe import Step # Import the wrapper class for pipeline steps
# Echo pipeline version and CRDS context in use
print("JWST Calibration Pipeline Version = {}".format(jwst.__version__))
print("Using CRDS Context = {}".format(crds.get_context_name('jwst')))
JWST Calibration Pipeline Version = 1.20.2
CRDS - INFO - Calibration SW Found: jwst 1.20.2 (/usr/share/miniconda/lib/python3.13/site-packages/jwst-1.20.2.dist-info)
Using CRDS Context = jwst_1464.pmap
Define convenience functions#
Define a convenience function to select only files of a given coronagraph mask/filter from an input set
# Define a convenience function to select only files of a given coronagraph mask/filter from an input set
def select_mask_filter_files(files, use_mask, use_filter):
"""
Filter FITS files based on mask and filter criteria from their headers.
Parameters:
-----------
files : array-like
List of FITS file paths to process
use_mask : str
Mask value to match in FITS header 'CORONMSK' key
use_filter : str
Filter value to match in FITS header 'FILTER' key
Returns:
--------
numpy.ndarray
Filtered array of file paths matching the criteria
"""
# Make paths absolute paths
for i in range(len(files)):
files[i] = os.path.abspath(files[i])
# Convert files to numpy array if it isn't already
files = np.asarray(files)
# If either mask or filter is empty, return all files
if not use_mask or not use_filter:
return files
try:
# Initialize boolean array for keeping track of matches
keep = np.zeros(len(files), dtype=bool)
# Process each file
for i in range(len(files)):
try:
with fits.open(files[i]) as hdu:
hdu.verify()
hdr = hdu[0].header
# Check if requred header keywords exist
if ('CORONMSK' in hdr and 'FILTER' in hdr):
if hdr['CORONMSK'] == use_mask and hdr['FILTER'] == use_filter:
keep[i] = True
files[i] = os.path.abspath(files[i])
except (OSError, ValueError) as e:
print(f" Warning: could not process file {files[i]}: {str(e)}")
# Return filtered files
indx = np.where(keep)
return files[indx]
except Exception as e:
print(f"Error processing files: {str(e)}")
return files # Return original array in case of failure
# Start a timer to keep track of runtime
time0 = time.perf_counter()
3.-Demo Mode Setup (ignore if not using demo data)#
If running in demonstration mode, set up the program information to
retrieve the uncalibrated data automatically from MAST using
astroquery.
MAST allows for flexibility of searching by the proposal ID and the
observation ID instead of just filenames.
For illustrative purposes, we focus on data taken through the MIRI
F1550C filter
and start with uncalibrated raw data products (uncal.fits). The files use the following naming schema:
jw01386<obs>001_04101_0000<dith>_mirimage_uncal.fits, where obs refers to the observation number and dith refers to the
dither step number.
More information about the JWST file naming conventions can be found at: https://jwst-pipeline.readthedocs.io/en/latest/jwst/data_products/file_naming.html
# Set up the program information and paths for demo program
if demo_mode:
print('Running in demonstration mode and will download example data from MAST!')
program = "01386"
sci_r1_observtn = "008"
sci_r2_observtn = "009"
ref_targ_observtn = "007"
bg_sci_observtn = "030"
bg_ref_targ_observtn = "031"
# ----------Define the base and observation directories----------
basedir = os.path.join('.', 'miri_coro_demo_data')
download_dir = basedir
sci_r1_dir = os.path.join(basedir, 'Obs' + sci_r1_observtn)
sci_r2_dir = os.path.join(basedir, 'Obs' + sci_r2_observtn)
ref_targ_dir = os.path.join(basedir, 'Obs' + ref_targ_observtn)
bg_sci_dir = os.path.join(basedir, 'Obs' + bg_sci_observtn)
bg_ref_targ_dir = os.path.join(basedir, 'Obs' + bg_ref_targ_observtn)
uncal_sci_r1_dir = os.path.join(sci_r1_dir, 'uncal')
uncal_sci_r2_dir = os.path.join(sci_r2_dir, 'uncal')
uncal_ref_targ_dir = os.path.join(ref_targ_dir, 'uncal')
uncal_bg_sci_dir = os.path.join(bg_sci_dir, 'uncal')
uncal_bg_ref_targ_dir = os.path.join(bg_ref_targ_dir, 'uncal')
# Ensure filepaths for input data exist
input_dirs = [uncal_sci_r1_dir, uncal_sci_r2_dir, uncal_ref_targ_dir, uncal_bg_sci_dir, uncal_bg_ref_targ_dir]
for dir in input_dirs:
if not os.path.exists(dir):
os.makedirs(dir)
Running in demonstration mode and will download example data from MAST!
Identify list of uncalibrated files associated with visits.
# Obtain a list of observation IDs for the specified demo program
if demo_mode:
obs_id_table = Observations.query_criteria(instrument_name=["MIRI/CORON"],
provenance_name=["CALJWST"],
proposal_id=[program])
# Turn the list of visits into a list of uncalibrated data files
if demo_mode:
# Define types of files to select
file_dict = {'uncal': {'product_type': 'SCIENCE', 'productSubGroupDescription': 'UNCAL', 'calib_level': [1]}}
# Loop over visits identifying uncalibrated files that are associated with them
files_to_download = []
for exposure in (obs_id_table):
products = Observations.get_product_list(exposure)
for filetype, query_dict in file_dict.items():
filtered_products = Observations.filter_products(products, productType=query_dict['product_type'],
productSubGroupDescription=query_dict['productSubGroupDescription'],
calib_level=query_dict['calib_level'])
files_to_download.extend(filtered_products['dataURI'])
# Cull to a unique list of files for each observation type
# Science roll 1
sci_r1_files_to_download = []
sci_r1_files_to_download = np.unique([i for i in files_to_download if str(program + sci_r1_observtn) in i])
# Science roll 2
sci_r2_files_to_download = []
sci_r2_files_to_download = np.unique([i for i in files_to_download if str(program + sci_r2_observtn) in i])
# PSF Reference taraget data
ref_targ_files_to_download = []
ref_targ_files_to_download = np.unique([i for i in files_to_download if str(program + ref_targ_observtn) in i])
# Background files (science assoc.)
bg_sci_files_to_download = []
bg_sci_files_to_download = np.unique([i for i in files_to_download if str(program + bg_sci_observtn) in i])
# Background files (reference target assoc.)
bg_ref_targ_files_to_download = []
bg_ref_targ_files_to_download = np.unique([i for i in files_to_download if str(program + bg_ref_targ_observtn) in i])
print("Science files selected for downloading: ", len(sci_r1_files_to_download) + len(sci_r1_files_to_download))
print("PSF Reference target files selected for downloading: ", len(ref_targ_files_to_download))
print("Background selected for downloading: ", len(bg_sci_files_to_download) + len(bg_ref_targ_files_to_download))
Science files selected for downloading: 6
PSF Reference target files selected for downloading: 11
Background selected for downloading: 4
For the demo example, there should be 6 Science files, 11 PSF Reference files and 4 Background files selected for downloading.
Download all the uncal files and place them into the appropriate directories.
if demo_mode:
for filename in sci_r1_files_to_download:
sci_r1_manifest = Observations.download_file(filename, local_path=os.path.join(uncal_sci_r1_dir, Path(filename).name))
for filename in sci_r2_files_to_download:
sci_r2_manifest = Observations.download_file(filename, local_path=os.path.join(uncal_sci_r2_dir, Path(filename).name))
for filename in ref_targ_files_to_download:
ref_targ_manifest = Observations.download_file(filename, local_path=os.path.join(uncal_ref_targ_dir, Path(filename).name))
for filename in bg_sci_files_to_download:
bg_manifest = Observations.download_file(filename, local_path=os.path.join(uncal_bg_sci_dir, Path(filename).name))
for filename in bg_ref_targ_files_to_download:
bg_ref_targ_manifest = Observations.download_file(filename, local_path=os.path.join(uncal_bg_ref_targ_dir, Path(filename).name))
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386008001_02101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs008/uncal/jw01386008001_02101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386008001_02101_00002_mirimage_uncal.fits to ./miri_coro_demo_data/Obs008/uncal/jw01386008001_02101_00002_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386008001_04101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs008/uncal/jw01386008001_04101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386009001_02101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs009/uncal/jw01386009001_02101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386009001_02101_00002_mirimage_uncal.fits to ./miri_coro_demo_data/Obs009/uncal/jw01386009001_02101_00002_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386009001_04101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs009/uncal/jw01386009001_04101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_02101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_02101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_02101_00002_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_02101_00002_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00002_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00002_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00003_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00003_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00004_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00004_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00005_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00005_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00006_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00006_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00007_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00007_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00008_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00008_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386007001_04101_00009_mirimage_uncal.fits to ./miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00009_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386030001_02101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs030/uncal/jw01386030001_02101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386030001_03101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs030/uncal/jw01386030001_03101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386031001_02101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs031/uncal/jw01386031001_02101_00001_mirimage_uncal.fits ...
[Done]
Downloading URL https://mast.stsci.edu/api/v0.1/Download/file?uri=mast:JWST/product/jw01386031001_03101_00001_mirimage_uncal.fits to ./miri_coro_demo_data/Obs031/uncal/jw01386031001_03101_00001_mirimage_uncal.fits ...
[Done]
4.-Directory Setup#
Set up detailed paths to input/output stages here. We will set up individual stage1/ and stage2/ sub directories for each observation, but a single stage3/ directory for the combined calwebb_coron3 output products.
# Define output subdirectories to keep science data products organized
# Sci Roll 1
uncal_sci_r1_dir = os.path.join(sci_r1_dir, 'uncal') # uncal inputs go here
det1_sci_r1_dir = os.path.join(sci_r1_dir, 'stage1') # calwebb_detector1 pipeline outputs will go here
image2_sci_r1_dir = os.path.join(sci_r1_dir, 'stage2') # calwebb_image2 pipeline outputs will go here
# Sci Roll 2
uncal_sci_r2_dir = os.path.join(sci_r2_dir, 'uncal') # uncal inputs go here
det1_sci_r2_dir = os.path.join(sci_r2_dir, 'stage1') # calwebb_detector1 pipeline outputs will go here
image2_sci_r2_dir = os.path.join(sci_r2_dir, 'stage2') # calwebb_image2 pipeline outputs will go here
# Define output subdirectories to keep PSF reference target data products organized
uncal_ref_targ_dir = os.path.join(ref_targ_dir, 'uncal') # uncal inputs go here
det1_ref_targ_dir = os.path.join(ref_targ_dir, 'stage1') # calwebb_detector1 pipeline outputs will go here
image2_ref_targ_dir = os.path.join(ref_targ_dir, 'stage2') # calwebb_image2 pipeline outputs will go here
# Define output subdirectories to keep background data products organized
# Sci Bkg
uncal_bg_sci_dir = os.path.join(bg_sci_dir, 'uncal') # uncal inputs go here
det1_bg_sci_dir = os.path.join(bg_sci_dir, 'stage1') # calwebb_detector1 pipeline outputs will go here
image2_bg_sci_dir = os.path.join(bg_sci_dir, 'stage2') # calwebb_image2 pipeline outputs will go here
# Ref target Bkg
uncal_bg_ref_targ_dir = os.path.join(bg_ref_targ_dir, 'uncal') # uncal inputs go here
det1_bg_ref_targ_dir = os.path.join(bg_ref_targ_dir, 'stage1') # calwebb_detector1 pipeline outputs will go here
image2_bg_ref_targ_dir = os.path.join(bg_ref_targ_dir, 'stage2') # calwebb_image2 pipeline outputs will go here
coron3_dir = os.path.join(basedir, 'stage3')
# We need to check that the desired output directories exist, and if not create them
det1_dirs = [det1_sci_r1_dir, det1_sci_r2_dir, det1_ref_targ_dir, det1_bg_sci_dir, det1_bg_ref_targ_dir]
image2_dirs = [image2_sci_r1_dir, image2_sci_r2_dir, image2_ref_targ_dir, image2_bg_sci_dir, image2_bg_ref_targ_dir]
for dir in det1_dirs:
if not os.path.exists(dir):
os.makedirs(dir)
for dir in image2_dirs:
if not os.path.exists(dir):
os.makedirs(dir)
if not os.path.exists(coron3_dir):
os.makedirs(coron3_dir)
# Print out the time benchmark
time1 = time.perf_counter()
print(f"Runtime so far: {time1 - time0:0.4f} seconds")
Runtime so far: 458.7402 seconds
5.-Detector1 Pipeline#
In this section, we process our uncalibrated data through the calwebb_detector1 pipeline to create Stage 1 data products. For coronagraphic exposures, these data products include a *_rate.fits file (a 2D countrate product, based on averaging over all integrations in the exposure), but specifically also a *_rateints.fits file, a 3D countrate product, that contains the individual results of each integration, wherein 2D countrate images for each integration are stacked along the 3rd axis of the data cubes (ncols x nrows x nints). These data products have units of DN/s.
See https://jwst-docs.stsci.edu/jwst-science-calibration-pipeline/stages-of-jwst-data-processing/calwebb_detector1
By default, all steps in the calwebb_detector1 are run for MIRI except: the ipc and charge_migration steps. There are also several steps performed for MIRI data that are not performed for other instruments. These include: emicorr, firstframe, lastframe, reset and rscd.
E.g., turn on detection of cosmic ray showers.
# Set up a dictionary to define how the Detector1 pipeline should be configured
# Boilerplate dictionary setup
det1dict = {}
det1dict['group_scale'], det1dict['dq_init'], det1dict['emicorr'], det1dict['saturation'] = {}, {}, {}, {}
det1dict['firstframe'], det1dict['lastframe'], det1dict['reset'], det1dict['linearity'], det1dict['rscd'] = {}, {}, {}, {}, {}
det1dict['dark_current'], det1dict['refpix'], det1dict['jump'], det1dict['ramp_fit'], det1dict['gain_scale'] = {}, {}, {}, {}, {}
det1dict['clean_flicker_noise'] = {}
# Overrides for whether or not certain steps should be skipped (example)
# skipping refpix step
#det1dict['refpix']['skip'] = True
# Overrides for various reference files
# Files should be in the base local directory or provide full path
#det1dict['dq_init']['override_mask'] = 'myfile.fits' # Bad pixel mask
#det1dict['saturation']['override_saturation'] = 'myfile.fits' # Saturation
#det1dict['reset']['override_reset'] = 'myfile.fits' # Reset
#det1dict['linearity']['override_linearity'] = 'myfile.fits' # Linearity
#det1dict['rscd']['override_rscd'] = 'myfile.fits' # RSCD
#det1dict['dark_current']['override_dark'] = 'myfile.fits' # Dark current subtraction
#det1dict['jump']['override_gain'] = 'myfile.fits' # Gain used by jump step
#det1dict['ramp_fit']['override_gain'] = 'myfile.fits' # Gain used by ramp fitting step
#det1dict['jump']['override_readnoise'] = 'myfile.fits' # Read noise used by jump step
#det1dict['ramp_fit']['override_readnoise'] = 'myfile.fits' # Read noise used by ramp fitting step
# Turn on multi-core processing (off by default). Choose what fraction of cores to use (quarter, half, or all)
det1dict['jump']['maximum_cores'] = 'half'
# Save the frame-averaged dark data created during the dark current subtraction step
#det1dict['dark_current']['dark_output'] = 'dark.fits' # Frame-averaged dark
# Turn on detection of cosmic ray showers (off by default)
#det1dict['jump']['find_showers'] = True
For more information see Tips and Trick for working with the JWST Pipeline
# Define a new step called XplyStep that multiplies everything by 1.0
# I.e., it does nothing, but could be changed to do something more interesting.
class XplyStep(Step):
spec = '''
'''
class_alias = 'xply'
def process(self, input_data):
with datamodels.open(input_data) as model:
result = model.copy()
sci = result.data
sci = sci * 1.0
result.data = sci
self.log.info('Multiplied everything by one in custom step!')
return result
# And here we'll insert it into our pipeline dictionary to be run at the end right after the gain_scale step
det1dict['gain_scale']['post_hooks'] = [XplyStep]
Calibrating Science Files#
Look for input science files and run calwebb_detector1 pipeline using the call method. For the demo example there should be 2 input science files, one for the observation at roll 1 (Obs 8) and one for the observation at roll 2 (Obs 9).
uncal_sci_r1_dir
'./miri_coro_demo_data/Obs008/uncal'
# Look for input files of the form *uncal.fits from the science observation
sstring1 = os.path.join(uncal_sci_r1_dir, 'jw*mirimage*uncal.fits')
sstring2 = os.path.join(uncal_sci_r2_dir, 'jw*mirimage*uncal.fits')
uncal_sci_r1_files = sorted(glob.glob(sstring1))
uncal_sci_r2_files = sorted(glob.glob(sstring2))
# Check that these are the correct mask/filter to use
uncal_sci_r1_files = select_mask_filter_files(uncal_sci_r1_files, use_mask, use_filter)
uncal_sci_r2_files = select_mask_filter_files(uncal_sci_r2_files, use_mask, use_filter)
print('Found ' + str((len(uncal_sci_r1_files) + len(uncal_sci_r2_files))) + ' science input files')
Found 2 science input files
# Run the pipeline on these input files by a simple loop over files using
# our custom parameter dictionary
if dodet1:
for file in uncal_sci_r1_files:
Detector1Pipeline.call(file, steps=det1dict, save_results=True, output_dir=det1_sci_r1_dir)
for file in uncal_sci_r2_files:
Detector1Pipeline.call(file, steps=det1dict, save_results=True, output_dir=det1_sci_r2_dir)
else:
print('Skipping Detector1 processing for SCI data')
2025-12-18 19:33:57,811 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_system_datalvl_0002.rmap 694 bytes (1 / 212 files) (0 / 762.3 K bytes)
2025-12-18 19:33:57,921 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_system_calver_0063.rmap 5.7 K bytes (2 / 212 files) (694 / 762.3 K bytes)
2025-12-18 19:33:58,032 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_system_0058.imap 385 bytes (3 / 212 files) (6.4 K / 762.3 K bytes)
2025-12-18 19:33:58,126 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_wavelengthrange_0024.rmap 1.4 K bytes (4 / 212 files) (6.8 K / 762.3 K bytes)
2025-12-18 19:33:58,251 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_wavecorr_0005.rmap 884 bytes (5 / 212 files) (8.2 K / 762.3 K bytes)
2025-12-18 19:33:58,341 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_superbias_0087.rmap 38.3 K bytes (6 / 212 files) (9.0 K / 762.3 K bytes)
2025-12-18 19:33:58,458 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_sirskernel_0002.rmap 704 bytes (7 / 212 files) (47.3 K / 762.3 K bytes)
2025-12-18 19:33:58,593 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_sflat_0027.rmap 20.6 K bytes (8 / 212 files) (48.0 K / 762.3 K bytes)
2025-12-18 19:33:58,703 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_saturation_0018.rmap 2.0 K bytes (9 / 212 files) (68.7 K / 762.3 K bytes)
2025-12-18 19:33:58,810 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_refpix_0015.rmap 1.6 K bytes (10 / 212 files) (70.7 K / 762.3 K bytes)
2025-12-18 19:33:58,928 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_readnoise_0025.rmap 2.6 K bytes (11 / 212 files) (72.2 K / 762.3 K bytes)
2025-12-18 19:33:59,017 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_psf_0002.rmap 687 bytes (12 / 212 files) (74.8 K / 762.3 K bytes)
2025-12-18 19:33:59,531 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pictureframe_0001.rmap 675 bytes (13 / 212 files) (75.5 K / 762.3 K bytes)
2025-12-18 19:33:59,623 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_photom_0013.rmap 958 bytes (14 / 212 files) (76.2 K / 762.3 K bytes)
2025-12-18 19:33:59,722 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pathloss_0011.rmap 1.2 K bytes (15 / 212 files) (77.1 K / 762.3 K bytes)
2025-12-18 19:33:59,818 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-whitelightstep_0001.rmap 777 bytes (16 / 212 files) (78.3 K / 762.3 K bytes)
2025-12-18 19:33:59,916 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-spec2pipeline_0013.rmap 2.1 K bytes (17 / 212 files) (79.1 K / 762.3 K bytes)
2025-12-18 19:34:00,004 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-resamplespecstep_0002.rmap 709 bytes (18 / 212 files) (81.2 K / 762.3 K bytes)
2025-12-18 19:34:00,096 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-refpixstep_0003.rmap 910 bytes (19 / 212 files) (81.9 K / 762.3 K bytes)
2025-12-18 19:34:00,189 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-outlierdetectionstep_0005.rmap 1.1 K bytes (20 / 212 files) (82.8 K / 762.3 K bytes)
2025-12-18 19:34:00,279 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-jumpstep_0005.rmap 810 bytes (21 / 212 files) (84.0 K / 762.3 K bytes)
2025-12-18 19:34:00,371 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-image2pipeline_0008.rmap 1.0 K bytes (22 / 212 files) (84.8 K / 762.3 K bytes)
2025-12-18 19:34:00,472 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-detector1pipeline_0003.rmap 1.1 K bytes (23 / 212 files) (85.8 K / 762.3 K bytes)
2025-12-18 19:34:00,562 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-darkpipeline_0003.rmap 872 bytes (24 / 212 files) (86.8 K / 762.3 K bytes)
2025-12-18 19:34:00,661 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_pars-darkcurrentstep_0003.rmap 1.8 K bytes (25 / 212 files) (87.7 K / 762.3 K bytes)
2025-12-18 19:34:00,754 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_ote_0030.rmap 1.3 K bytes (26 / 212 files) (89.5 K / 762.3 K bytes)
2025-12-18 19:34:00,846 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_msaoper_0017.rmap 1.6 K bytes (27 / 212 files) (90.8 K / 762.3 K bytes)
2025-12-18 19:34:00,941 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_msa_0027.rmap 1.3 K bytes (28 / 212 files) (92.4 K / 762.3 K bytes)
2025-12-18 19:34:01,037 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_mask_0044.rmap 4.3 K bytes (29 / 212 files) (93.6 K / 762.3 K bytes)
2025-12-18 19:34:01,132 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_linearity_0017.rmap 1.6 K bytes (30 / 212 files) (97.9 K / 762.3 K bytes)
2025-12-18 19:34:01,218 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_ipc_0006.rmap 876 bytes (31 / 212 files) (99.5 K / 762.3 K bytes)
2025-12-18 19:34:01,311 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_ifuslicer_0017.rmap 1.5 K bytes (32 / 212 files) (100.4 K / 762.3 K bytes)
2025-12-18 19:34:01,404 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_ifupost_0019.rmap 1.5 K bytes (33 / 212 files) (101.9 K / 762.3 K bytes)
2025-12-18 19:34:01,492 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_ifufore_0017.rmap 1.5 K bytes (34 / 212 files) (103.4 K / 762.3 K bytes)
2025-12-18 19:34:01,580 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_gain_0023.rmap 1.8 K bytes (35 / 212 files) (104.9 K / 762.3 K bytes)
2025-12-18 19:34:01,710 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_fpa_0028.rmap 1.3 K bytes (36 / 212 files) (106.7 K / 762.3 K bytes)
2025-12-18 19:34:01,820 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_fore_0026.rmap 5.0 K bytes (37 / 212 files) (107.9 K / 762.3 K bytes)
2025-12-18 19:34:01,911 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_flat_0015.rmap 3.8 K bytes (38 / 212 files) (112.9 K / 762.3 K bytes)
2025-12-18 19:34:02,003 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_fflat_0028.rmap 7.2 K bytes (39 / 212 files) (116.7 K / 762.3 K bytes)
2025-12-18 19:34:02,098 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_extract1d_0018.rmap 2.3 K bytes (40 / 212 files) (123.9 K / 762.3 K bytes)
2025-12-18 19:34:02,187 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_disperser_0028.rmap 5.7 K bytes (41 / 212 files) (126.2 K / 762.3 K bytes)
2025-12-18 19:34:02,280 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_dflat_0007.rmap 1.1 K bytes (42 / 212 files) (131.9 K / 762.3 K bytes)
2025-12-18 19:34:02,381 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_dark_0083.rmap 36.4 K bytes (43 / 212 files) (133.0 K / 762.3 K bytes)
2025-12-18 19:34:02,495 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_cubepar_0015.rmap 966 bytes (44 / 212 files) (169.4 K / 762.3 K bytes)
2025-12-18 19:34:02,585 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_collimator_0026.rmap 1.3 K bytes (45 / 212 files) (170.4 K / 762.3 K bytes)
2025-12-18 19:34:02,676 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_camera_0026.rmap 1.3 K bytes (46 / 212 files) (171.7 K / 762.3 K bytes)
2025-12-18 19:34:02,773 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_barshadow_0007.rmap 1.8 K bytes (47 / 212 files) (173.0 K / 762.3 K bytes)
2025-12-18 19:34:02,882 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_area_0018.rmap 6.3 K bytes (48 / 212 files) (174.8 K / 762.3 K bytes)
2025-12-18 19:34:02,992 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_apcorr_0009.rmap 5.6 K bytes (49 / 212 files) (181.1 K / 762.3 K bytes)
2025-12-18 19:34:03,086 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nirspec_0420.imap 5.8 K bytes (50 / 212 files) (186.7 K / 762.3 K bytes)
2025-12-18 19:34:03,185 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_wavelengthrange_0008.rmap 897 bytes (51 / 212 files) (192.5 K / 762.3 K bytes)
2025-12-18 19:34:03,275 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_trappars_0004.rmap 753 bytes (52 / 212 files) (193.4 K / 762.3 K bytes)
2025-12-18 19:34:03,369 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_trapdensity_0005.rmap 705 bytes (53 / 212 files) (194.1 K / 762.3 K bytes)
2025-12-18 19:34:03,464 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_throughput_0005.rmap 1.3 K bytes (54 / 212 files) (194.8 K / 762.3 K bytes)
2025-12-18 19:34:03,557 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_superbias_0033.rmap 8.0 K bytes (55 / 212 files) (196.1 K / 762.3 K bytes)
2025-12-18 19:34:03,651 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_specwcs_0016.rmap 3.1 K bytes (56 / 212 files) (204.1 K / 762.3 K bytes)
2025-12-18 19:34:03,755 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_specprofile_0008.rmap 2.4 K bytes (57 / 212 files) (207.2 K / 762.3 K bytes)
2025-12-18 19:34:03,847 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_speckernel_0006.rmap 1.0 K bytes (58 / 212 files) (209.6 K / 762.3 K bytes)
2025-12-18 19:34:03,938 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_sirskernel_0002.rmap 700 bytes (59 / 212 files) (210.6 K / 762.3 K bytes)
2025-12-18 19:34:04,027 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_saturation_0015.rmap 829 bytes (60 / 212 files) (211.3 K / 762.3 K bytes)
2025-12-18 19:34:04,131 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_readnoise_0011.rmap 987 bytes (61 / 212 files) (212.1 K / 762.3 K bytes)
2025-12-18 19:34:04,237 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_photom_0037.rmap 1.3 K bytes (62 / 212 files) (213.1 K / 762.3 K bytes)
2025-12-18 19:34:04,331 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_persat_0007.rmap 674 bytes (63 / 212 files) (214.4 K / 762.3 K bytes)
2025-12-18 19:34:04,421 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pathloss_0003.rmap 758 bytes (64 / 212 files) (215.1 K / 762.3 K bytes)
2025-12-18 19:34:04,513 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pastasoss_0005.rmap 818 bytes (65 / 212 files) (215.8 K / 762.3 K bytes)
2025-12-18 19:34:04,605 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-undersamplecorrectionstep_0001.rmap 904 bytes (66 / 212 files) (216.6 K / 762.3 K bytes)
2025-12-18 19:34:04,714 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-tweakregstep_0012.rmap 3.1 K bytes (67 / 212 files) (217.5 K / 762.3 K bytes)
2025-12-18 19:34:04,867 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-spec2pipeline_0009.rmap 1.2 K bytes (68 / 212 files) (220.7 K / 762.3 K bytes)
2025-12-18 19:34:04,964 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-sourcecatalogstep_0002.rmap 2.3 K bytes (69 / 212 files) (221.9 K / 762.3 K bytes)
2025-12-18 19:34:06,060 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-resamplestep_0002.rmap 687 bytes (70 / 212 files) (224.2 K / 762.3 K bytes)
2025-12-18 19:34:06,155 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-outlierdetectionstep_0004.rmap 2.7 K bytes (71 / 212 files) (224.9 K / 762.3 K bytes)
2025-12-18 19:34:06,245 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-jumpstep_0007.rmap 6.4 K bytes (72 / 212 files) (227.6 K / 762.3 K bytes)
2025-12-18 19:34:06,332 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-image2pipeline_0005.rmap 1.0 K bytes (73 / 212 files) (233.9 K / 762.3 K bytes)
2025-12-18 19:34:06,419 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-detector1pipeline_0002.rmap 1.0 K bytes (74 / 212 files) (235.0 K / 762.3 K bytes)
2025-12-18 19:34:06,511 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-darkpipeline_0002.rmap 868 bytes (75 / 212 files) (236.0 K / 762.3 K bytes)
2025-12-18 19:34:06,600 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-darkcurrentstep_0001.rmap 591 bytes (76 / 212 files) (236.9 K / 762.3 K bytes)
2025-12-18 19:34:06,693 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-chargemigrationstep_0005.rmap 5.7 K bytes (77 / 212 files) (237.5 K / 762.3 K bytes)
2025-12-18 19:34:06,781 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_pars-backgroundstep_0003.rmap 822 bytes (78 / 212 files) (243.1 K / 762.3 K bytes)
2025-12-18 19:34:06,875 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_nrm_0005.rmap 663 bytes (79 / 212 files) (243.9 K / 762.3 K bytes)
2025-12-18 19:34:06,962 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_mask_0024.rmap 1.5 K bytes (80 / 212 files) (244.6 K / 762.3 K bytes)
2025-12-18 19:34:07,057 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_linearity_0022.rmap 961 bytes (81 / 212 files) (246.1 K / 762.3 K bytes)
2025-12-18 19:34:07,159 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_ipc_0007.rmap 651 bytes (82 / 212 files) (247.0 K / 762.3 K bytes)
2025-12-18 19:34:07,249 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_gain_0011.rmap 797 bytes (83 / 212 files) (247.7 K / 762.3 K bytes)
2025-12-18 19:34:07,337 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_flat_0023.rmap 5.9 K bytes (84 / 212 files) (248.5 K / 762.3 K bytes)
2025-12-18 19:34:07,428 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_filteroffset_0010.rmap 853 bytes (85 / 212 files) (254.3 K / 762.3 K bytes)
2025-12-18 19:34:07,517 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_extract1d_0007.rmap 905 bytes (86 / 212 files) (255.2 K / 762.3 K bytes)
2025-12-18 19:34:07,611 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_drizpars_0004.rmap 519 bytes (87 / 212 files) (256.1 K / 762.3 K bytes)
2025-12-18 19:34:07,716 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_distortion_0025.rmap 3.4 K bytes (88 / 212 files) (256.6 K / 762.3 K bytes)
2025-12-18 19:34:07,805 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_dark_0037.rmap 8.1 K bytes (89 / 212 files) (260.1 K / 762.3 K bytes)
2025-12-18 19:34:07,892 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_bkg_0005.rmap 3.1 K bytes (90 / 212 files) (268.2 K / 762.3 K bytes)
2025-12-18 19:34:07,990 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_area_0014.rmap 2.7 K bytes (91 / 212 files) (271.2 K / 762.3 K bytes)
2025-12-18 19:34:08,079 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_apcorr_0010.rmap 4.3 K bytes (92 / 212 files) (273.9 K / 762.3 K bytes)
2025-12-18 19:34:08,166 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_abvegaoffset_0004.rmap 1.4 K bytes (93 / 212 files) (278.2 K / 762.3 K bytes)
2025-12-18 19:34:08,256 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_niriss_0292.imap 5.8 K bytes (94 / 212 files) (279.6 K / 762.3 K bytes)
2025-12-18 19:34:08,347 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_wavelengthrange_0011.rmap 996 bytes (95 / 212 files) (285.3 K / 762.3 K bytes)
2025-12-18 19:34:08,432 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_tsophot_0003.rmap 896 bytes (96 / 212 files) (286.3 K / 762.3 K bytes)
2025-12-18 19:34:08,521 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_trappars_0003.rmap 1.6 K bytes (97 / 212 files) (287.2 K / 762.3 K bytes)
2025-12-18 19:34:08,609 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_trapdensity_0003.rmap 1.6 K bytes (98 / 212 files) (288.8 K / 762.3 K bytes)
2025-12-18 19:34:08,724 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_superbias_0020.rmap 19.6 K bytes (99 / 212 files) (290.5 K / 762.3 K bytes)
2025-12-18 19:34:08,839 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_specwcs_0024.rmap 8.0 K bytes (100 / 212 files) (310.0 K / 762.3 K bytes)
2025-12-18 19:34:08,932 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_sirskernel_0003.rmap 671 bytes (101 / 212 files) (318.0 K / 762.3 K bytes)
2025-12-18 19:34:09,018 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_saturation_0011.rmap 2.8 K bytes (102 / 212 files) (318.7 K / 762.3 K bytes)
2025-12-18 19:34:09,103 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_regions_0002.rmap 725 bytes (103 / 212 files) (321.5 K / 762.3 K bytes)
2025-12-18 19:34:09,191 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_readnoise_0027.rmap 26.6 K bytes (104 / 212 files) (322.2 K / 762.3 K bytes)
2025-12-18 19:34:09,324 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_psfmask_0008.rmap 28.4 K bytes (105 / 212 files) (348.8 K / 762.3 K bytes)
2025-12-18 19:34:09,435 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_photom_0028.rmap 3.4 K bytes (106 / 212 files) (377.2 K / 762.3 K bytes)
2025-12-18 19:34:09,520 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_persat_0005.rmap 1.6 K bytes (107 / 212 files) (380.5 K / 762.3 K bytes)
2025-12-18 19:34:09,606 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-whitelightstep_0004.rmap 2.0 K bytes (108 / 212 files) (382.1 K / 762.3 K bytes)
2025-12-18 19:34:09,690 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-tweakregstep_0003.rmap 4.5 K bytes (109 / 212 files) (384.1 K / 762.3 K bytes)
2025-12-18 19:34:09,785 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-tsophotometrystep_0003.rmap 1.1 K bytes (110 / 212 files) (388.6 K / 762.3 K bytes)
2025-12-18 19:34:09,878 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-spec2pipeline_0009.rmap 984 bytes (111 / 212 files) (389.6 K / 762.3 K bytes)
2025-12-18 19:34:09,963 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-sourcecatalogstep_0002.rmap 4.6 K bytes (112 / 212 files) (390.6 K / 762.3 K bytes)
2025-12-18 19:34:10,058 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-resamplestep_0002.rmap 687 bytes (113 / 212 files) (395.3 K / 762.3 K bytes)
2025-12-18 19:34:10,156 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-outlierdetectionstep_0003.rmap 940 bytes (114 / 212 files) (396.0 K / 762.3 K bytes)
2025-12-18 19:34:10,242 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-jumpstep_0005.rmap 806 bytes (115 / 212 files) (396.9 K / 762.3 K bytes)
2025-12-18 19:34:10,336 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-image2pipeline_0004.rmap 1.1 K bytes (116 / 212 files) (397.7 K / 762.3 K bytes)
2025-12-18 19:34:10,422 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-detector1pipeline_0006.rmap 1.7 K bytes (117 / 212 files) (398.8 K / 762.3 K bytes)
2025-12-18 19:34:10,531 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-darkpipeline_0002.rmap 868 bytes (118 / 212 files) (400.6 K / 762.3 K bytes)
2025-12-18 19:34:10,623 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-darkcurrentstep_0001.rmap 618 bytes (119 / 212 files) (401.4 K / 762.3 K bytes)
2025-12-18 19:34:10,725 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_pars-backgroundstep_0003.rmap 822 bytes (120 / 212 files) (402.0 K / 762.3 K bytes)
2025-12-18 19:34:10,813 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_mask_0013.rmap 4.8 K bytes (121 / 212 files) (402.9 K / 762.3 K bytes)
2025-12-18 19:34:10,908 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_linearity_0011.rmap 2.4 K bytes (122 / 212 files) (407.6 K / 762.3 K bytes)
2025-12-18 19:34:10,995 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_ipc_0003.rmap 2.0 K bytes (123 / 212 files) (410.0 K / 762.3 K bytes)
2025-12-18 19:34:11,086 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_gain_0016.rmap 2.1 K bytes (124 / 212 files) (412.0 K / 762.3 K bytes)
2025-12-18 19:34:11,172 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_flat_0028.rmap 51.7 K bytes (125 / 212 files) (414.1 K / 762.3 K bytes)
2025-12-18 19:34:11,298 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_filteroffset_0004.rmap 1.4 K bytes (126 / 212 files) (465.8 K / 762.3 K bytes)
2025-12-18 19:34:11,385 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_extract1d_0005.rmap 1.2 K bytes (127 / 212 files) (467.2 K / 762.3 K bytes)
2025-12-18 19:34:11,470 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_drizpars_0001.rmap 519 bytes (128 / 212 files) (468.4 K / 762.3 K bytes)
2025-12-18 19:34:11,563 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_distortion_0034.rmap 53.4 K bytes (129 / 212 files) (468.9 K / 762.3 K bytes)
2025-12-18 19:34:11,695 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_dark_0049.rmap 29.6 K bytes (130 / 212 files) (522.3 K / 762.3 K bytes)
2025-12-18 19:34:11,805 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_bkg_0002.rmap 7.0 K bytes (131 / 212 files) (551.9 K / 762.3 K bytes)
2025-12-18 19:34:11,899 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_area_0012.rmap 33.5 K bytes (132 / 212 files) (558.9 K / 762.3 K bytes)
2025-12-18 19:34:12,008 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_apcorr_0008.rmap 4.3 K bytes (133 / 212 files) (592.4 K / 762.3 K bytes)
2025-12-18 19:34:12,105 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_abvegaoffset_0003.rmap 1.3 K bytes (134 / 212 files) (596.6 K / 762.3 K bytes)
2025-12-18 19:34:12,194 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_nircam_0333.imap 5.7 K bytes (135 / 212 files) (597.9 K / 762.3 K bytes)
2025-12-18 19:34:12,284 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_wavelengthrange_0029.rmap 1.0 K bytes (136 / 212 files) (603.6 K / 762.3 K bytes)
2025-12-18 19:34:12,376 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_tsophot_0004.rmap 882 bytes (137 / 212 files) (604.6 K / 762.3 K bytes)
2025-12-18 19:34:12,463 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_straymask_0009.rmap 987 bytes (138 / 212 files) (605.5 K / 762.3 K bytes)
2025-12-18 19:34:12,555 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_specwcs_0047.rmap 5.9 K bytes (139 / 212 files) (606.5 K / 762.3 K bytes)
2025-12-18 19:34:12,642 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_saturation_0015.rmap 1.2 K bytes (140 / 212 files) (612.4 K / 762.3 K bytes)
2025-12-18 19:34:12,749 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_rscd_0008.rmap 1.0 K bytes (141 / 212 files) (613.6 K / 762.3 K bytes)
2025-12-18 19:34:12,837 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_resol_0006.rmap 790 bytes (142 / 212 files) (614.6 K / 762.3 K bytes)
2025-12-18 19:34:12,929 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_reset_0026.rmap 3.9 K bytes (143 / 212 files) (615.4 K / 762.3 K bytes)
2025-12-18 19:34:13,022 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_regions_0036.rmap 4.4 K bytes (144 / 212 files) (619.3 K / 762.3 K bytes)
2025-12-18 19:34:13,111 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_readnoise_0023.rmap 1.6 K bytes (145 / 212 files) (623.6 K / 762.3 K bytes)
2025-12-18 19:34:13,201 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_psfmask_0009.rmap 2.1 K bytes (146 / 212 files) (625.3 K / 762.3 K bytes)
2025-12-18 19:34:13,289 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_psf_0008.rmap 2.6 K bytes (147 / 212 files) (627.4 K / 762.3 K bytes)
2025-12-18 19:34:13,380 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_photom_0062.rmap 3.7 K bytes (148 / 212 files) (630.0 K / 762.3 K bytes)
2025-12-18 19:34:13,467 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pathloss_0005.rmap 866 bytes (149 / 212 files) (633.8 K / 762.3 K bytes)
2025-12-18 19:34:13,554 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-whitelightstep_0003.rmap 912 bytes (150 / 212 files) (634.6 K / 762.3 K bytes)
2025-12-18 19:34:13,641 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-tweakregstep_0003.rmap 1.8 K bytes (151 / 212 files) (635.5 K / 762.3 K bytes)
2025-12-18 19:34:13,734 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-tsophotometrystep_0003.rmap 2.7 K bytes (152 / 212 files) (637.4 K / 762.3 K bytes)
2025-12-18 19:34:13,827 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-spec3pipeline_0011.rmap 886 bytes (153 / 212 files) (640.0 K / 762.3 K bytes)
2025-12-18 19:34:13,930 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-spec2pipeline_0013.rmap 1.4 K bytes (154 / 212 files) (640.9 K / 762.3 K bytes)
2025-12-18 19:34:14,026 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-sourcecatalogstep_0003.rmap 1.9 K bytes (155 / 212 files) (642.3 K / 762.3 K bytes)
2025-12-18 19:34:14,115 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-resamplestep_0002.rmap 677 bytes (156 / 212 files) (644.2 K / 762.3 K bytes)
2025-12-18 19:34:14,206 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-resamplespecstep_0002.rmap 706 bytes (157 / 212 files) (644.9 K / 762.3 K bytes)
2025-12-18 19:34:14,301 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-outlierdetectionstep_0020.rmap 3.4 K bytes (158 / 212 files) (645.6 K / 762.3 K bytes)
2025-12-18 19:34:14,388 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-jumpstep_0011.rmap 1.6 K bytes (159 / 212 files) (649.0 K / 762.3 K bytes)
2025-12-18 19:34:14,477 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-image2pipeline_0010.rmap 1.1 K bytes (160 / 212 files) (650.6 K / 762.3 K bytes)
2025-12-18 19:34:14,566 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-extract1dstep_0003.rmap 807 bytes (161 / 212 files) (651.7 K / 762.3 K bytes)
2025-12-18 19:34:14,654 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-emicorrstep_0003.rmap 796 bytes (162 / 212 files) (652.5 K / 762.3 K bytes)
2025-12-18 19:34:14,749 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-detector1pipeline_0010.rmap 1.6 K bytes (163 / 212 files) (653.3 K / 762.3 K bytes)
2025-12-18 19:34:14,841 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-darkpipeline_0002.rmap 860 bytes (164 / 212 files) (654.9 K / 762.3 K bytes)
2025-12-18 19:34:14,927 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-darkcurrentstep_0002.rmap 683 bytes (165 / 212 files) (655.7 K / 762.3 K bytes)
2025-12-18 19:34:15,017 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_pars-backgroundstep_0003.rmap 814 bytes (166 / 212 files) (656.4 K / 762.3 K bytes)
2025-12-18 19:34:15,104 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_mrsxartcorr_0002.rmap 2.2 K bytes (167 / 212 files) (657.2 K / 762.3 K bytes)
2025-12-18 19:34:15,198 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_mrsptcorr_0005.rmap 2.0 K bytes (168 / 212 files) (659.4 K / 762.3 K bytes)
2025-12-18 19:34:15,287 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_mask_0032.rmap 7.3 K bytes (169 / 212 files) (661.3 K / 762.3 K bytes)
2025-12-18 19:34:15,375 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_linearity_0018.rmap 2.8 K bytes (170 / 212 files) (668.6 K / 762.3 K bytes)
2025-12-18 19:34:15,465 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_ipc_0008.rmap 700 bytes (171 / 212 files) (671.4 K / 762.3 K bytes)
2025-12-18 19:34:15,553 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_gain_0013.rmap 3.9 K bytes (172 / 212 files) (672.1 K / 762.3 K bytes)
2025-12-18 19:34:15,638 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_fringefreq_0003.rmap 1.4 K bytes (173 / 212 files) (676.1 K / 762.3 K bytes)
2025-12-18 19:34:15,724 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_fringe_0019.rmap 3.9 K bytes (174 / 212 files) (677.5 K / 762.3 K bytes)
2025-12-18 19:34:15,818 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_flat_0071.rmap 15.7 K bytes (175 / 212 files) (681.4 K / 762.3 K bytes)
2025-12-18 19:34:15,925 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_filteroffset_0027.rmap 2.1 K bytes (176 / 212 files) (697.1 K / 762.3 K bytes)
2025-12-18 19:34:16,012 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_extract1d_0021.rmap 1.4 K bytes (177 / 212 files) (699.2 K / 762.3 K bytes)
2025-12-18 19:34:16,102 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_emicorr_0004.rmap 663 bytes (178 / 212 files) (700.6 K / 762.3 K bytes)
2025-12-18 19:34:16,189 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_drizpars_0002.rmap 511 bytes (179 / 212 files) (701.3 K / 762.3 K bytes)
2025-12-18 19:34:16,275 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_distortion_0042.rmap 4.8 K bytes (180 / 212 files) (701.8 K / 762.3 K bytes)
2025-12-18 19:34:16,368 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_dark_0037.rmap 4.4 K bytes (181 / 212 files) (706.6 K / 762.3 K bytes)
2025-12-18 19:34:16,452 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_cubepar_0017.rmap 800 bytes (182 / 212 files) (711.0 K / 762.3 K bytes)
2025-12-18 19:34:16,535 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_bkg_0003.rmap 712 bytes (183 / 212 files) (711.8 K / 762.3 K bytes)
2025-12-18 19:34:16,619 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_area_0015.rmap 866 bytes (184 / 212 files) (712.5 K / 762.3 K bytes)
2025-12-18 19:34:16,703 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_apcorr_0022.rmap 5.0 K bytes (185 / 212 files) (713.3 K / 762.3 K bytes)
2025-12-18 19:34:16,787 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_abvegaoffset_0003.rmap 1.3 K bytes (186 / 212 files) (718.3 K / 762.3 K bytes)
2025-12-18 19:34:16,875 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_miri_0468.imap 5.9 K bytes (187 / 212 files) (719.6 K / 762.3 K bytes)
2025-12-18 19:34:16,963 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_trappars_0004.rmap 903 bytes (188 / 212 files) (725.5 K / 762.3 K bytes)
2025-12-18 19:34:17,051 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_trapdensity_0006.rmap 930 bytes (189 / 212 files) (726.4 K / 762.3 K bytes)
2025-12-18 19:34:17,138 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_superbias_0017.rmap 3.8 K bytes (190 / 212 files) (727.3 K / 762.3 K bytes)
2025-12-18 19:34:17,223 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_saturation_0009.rmap 779 bytes (191 / 212 files) (731.1 K / 762.3 K bytes)
2025-12-18 19:34:17,311 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_readnoise_0014.rmap 1.3 K bytes (192 / 212 files) (731.9 K / 762.3 K bytes)
2025-12-18 19:34:17,407 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_photom_0014.rmap 1.1 K bytes (193 / 212 files) (733.1 K / 762.3 K bytes)
2025-12-18 19:34:17,489 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_persat_0006.rmap 884 bytes (194 / 212 files) (734.2 K / 762.3 K bytes)
2025-12-18 19:34:17,579 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-tweakregstep_0002.rmap 850 bytes (195 / 212 files) (735.1 K / 762.3 K bytes)
2025-12-18 19:34:17,667 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-sourcecatalogstep_0001.rmap 636 bytes (196 / 212 files) (736.0 K / 762.3 K bytes)
2025-12-18 19:34:17,756 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-outlierdetectionstep_0001.rmap 654 bytes (197 / 212 files) (736.6 K / 762.3 K bytes)
2025-12-18 19:34:17,846 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-image2pipeline_0005.rmap 974 bytes (198 / 212 files) (737.3 K / 762.3 K bytes)
2025-12-18 19:34:17,934 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-detector1pipeline_0002.rmap 1.0 K bytes (199 / 212 files) (738.2 K / 762.3 K bytes)
2025-12-18 19:34:18,031 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_pars-darkpipeline_0002.rmap 856 bytes (200 / 212 files) (739.3 K / 762.3 K bytes)
2025-12-18 19:34:18,119 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_mask_0023.rmap 1.1 K bytes (201 / 212 files) (740.1 K / 762.3 K bytes)
2025-12-18 19:34:18,210 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_linearity_0015.rmap 925 bytes (202 / 212 files) (741.2 K / 762.3 K bytes)
2025-12-18 19:34:18,295 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_ipc_0003.rmap 614 bytes (203 / 212 files) (742.1 K / 762.3 K bytes)
2025-12-18 19:34:18,390 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_gain_0010.rmap 890 bytes (204 / 212 files) (742.7 K / 762.3 K bytes)
2025-12-18 19:34:18,478 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_flat_0009.rmap 1.1 K bytes (205 / 212 files) (743.6 K / 762.3 K bytes)
2025-12-18 19:34:18,571 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_distortion_0011.rmap 1.2 K bytes (206 / 212 files) (744.7 K / 762.3 K bytes)
2025-12-18 19:34:18,665 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_dark_0017.rmap 4.3 K bytes (207 / 212 files) (746.0 K / 762.3 K bytes)
2025-12-18 19:34:18,769 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_area_0010.rmap 1.2 K bytes (208 / 212 files) (750.3 K / 762.3 K bytes)
2025-12-18 19:34:18,862 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_apcorr_0004.rmap 4.0 K bytes (209 / 212 files) (751.4 K / 762.3 K bytes)
2025-12-18 19:34:18,946 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_abvegaoffset_0002.rmap 1.3 K bytes (210 / 212 files) (755.4 K / 762.3 K bytes)
2025-12-18 19:34:19,037 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_fgs_0124.imap 5.1 K bytes (211 / 212 files) (756.6 K / 762.3 K bytes)
2025-12-18 19:34:19,127 - CRDS - INFO - Fetching /home/runner/crds/mappings/jwst/jwst_1464.pmap 580 bytes (212 / 212 files) (761.7 K / 762.3 K bytes)
2025-12-18 19:34:19,763 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf 1.0 K bytes (1 / 1 files) (0 / 1.0 K bytes)
2025-12-18 19:34:19,854 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:34:19,872 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf 936 bytes (1 / 1 files) (0 / 936 bytes)
2025-12-18 19:34:19,961 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:34:19,971 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf 1.9 K bytes (1 / 1 files) (0 / 1.9 K bytes)
2025-12-18 19:34:20,061 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:34:20,073 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf 1.7 K bytes (1 / 1 files) (0 / 1.7 K bytes)
2025-12-18 19:34:20,161 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:34:20,179 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:34:20,180 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:34:20,181 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:34:20,182 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:34:20,182 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:34:20,183 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:34:20,184 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:34:20,185 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:34:20,186 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:34:20,186 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:34:20,187 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:34:20,188 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:34:20,189 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:34:20,189 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:34:20,190 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:34:20,191 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:34:20,192 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:34:20,193 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:34:20,194 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:34:20,195 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:34:20,196 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:34:20,330 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs008/uncal/jw01386008001_04101_00001_mirimage_uncal.fits'),).
2025-12-18 19:34:20,351 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs008/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:34:20,376 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386008001_04101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:34:20,379 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits 774.9 M bytes (1 / 9 files) (0 / 845.8 M bytes)
2025-12-18 19:34:32,244 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf 17.1 K bytes (2 / 9 files) (774.9 M / 845.8 M bytes)
2025-12-18 19:34:32,347 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits 12.7 M bytes (3 / 9 files) (775.0 M / 845.8 M bytes)
2025-12-18 19:34:32,698 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits 25.4 M bytes (4 / 9 files) (787.7 M / 845.8 M bytes)
2025-12-18 19:34:33,321 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits 4.3 M bytes (5 / 9 files) (813.0 M / 845.8 M bytes)
2025-12-18 19:34:33,658 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits 4.2 M bytes (6 / 9 files) (817.3 M / 845.8 M bytes)
2025-12-18 19:34:35,113 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits 15.8 M bytes (7 / 9 files) (821.5 M / 845.8 M bytes)
2025-12-18 19:34:35,527 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits 37.4 K bytes (8 / 9 files) (837.3 M / 845.8 M bytes)
2025-12-18 19:34:35,635 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits 8.5 M bytes (9 / 9 files) (837.3 M / 845.8 M bytes)
2025-12-18 19:34:36,073 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:34:36,074 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:34:36,074 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:34:36,075 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:34:36,075 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:34:36,076 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:34:36,076 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:34:36,077 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:34:36,078 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:34:36,078 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:34:36,079 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:34:36,079 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:34:36,079 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:34:36,080 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:34:38,652 - stpipe.step - INFO - Step group_scale running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:34:39,459 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:34:39,460 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:34:39,462 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:34:39,623 - stpipe.step - INFO - Step dq_init running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:34:39,634 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:34:40,461 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:34:40,462 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:34:40,472 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:34:40,476 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:34:42,464 - CRDS - INFO - Calibration SW Found: jwst 1.20.2 (/usr/share/miniconda/lib/python3.13/site-packages/jwst-1.20.2.dist-info)
2025-12-18 19:34:42,569 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:34:42,728 - stpipe.step - INFO - Step emicorr running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:34:43,533 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:34:43,551 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:34:43,552 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:34:43,553 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:34:43,553 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:34:43,554 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:35:57,492 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:35:57,686 - stpipe.step - INFO - Step saturation running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:35:58,492 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:35:58,493 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:35:58,517 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:35:58,518 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:35:58,526 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:35:58,529 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:35:58,536 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:36:05,825 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:36:06,059 - stcal.saturation.saturation - INFO - Detected 5 A/D floor pixels
2025-12-18 19:36:06,063 - stpipe.step - INFO - Step saturation done
2025-12-18 19:36:06,223 - stpipe.step - INFO - Step ipc running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:06,224 - stpipe.step - INFO - Step skipped.
2025-12-18 19:36:06,350 - stpipe.step - INFO - Step firstframe running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:07,176 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:36:07,178 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:36:07,338 - stpipe.step - INFO - Step lastframe running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:08,143 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:36:08,304 - stpipe.step - INFO - Step reset running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:09,129 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:36:09,152 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:09,153 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:09,155 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:09,236 - stpipe.step - INFO - Step reset done
2025-12-18 19:36:09,396 - stpipe.step - INFO - Step linearity running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:10,216 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:36:10,244 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:10,244 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:10,253 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:15,886 - stpipe.step - INFO - Step linearity done
2025-12-18 19:36:16,048 - stpipe.step - INFO - Step rscd running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:16,857 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:36:16,891 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:36:16,895 - stpipe.step - INFO - Step rscd done
2025-12-18 19:36:17,052 - stpipe.step - INFO - Step dark_current running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:17,064 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:36:18,028 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:18,029 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:18,033 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:36:18,034 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:36:18,035 - stcal.dark_current.dark_sub - INFO - Science data nints=60, ngroups=250, nframes=1, groupgap=0
2025-12-18 19:36:18,036 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:36:19,939 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:36:20,105 - stpipe.step - INFO - Step refpix running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:20,106 - stpipe.step - INFO - Step skipped.
2025-12-18 19:36:20,230 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:20,231 - stpipe.step - INFO - Step skipped.
2025-12-18 19:36:20,351 - stpipe.step - INFO - Step jump running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:21,161 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:36:21,162 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:36:21,170 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:36:21,173 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:36:21,200 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:36:21,205 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:36:22,432 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:36:22,433 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:36:51,369 - stcal.jump.jump - INFO - Total elapsed time = 28.9361 sec
2025-12-18 19:36:51,557 - jwst.jump.jump_step - INFO - The execution time in seconds: 31.198372
2025-12-18 19:36:51,560 - stpipe.step - INFO - Step jump done
2025-12-18 19:36:51,721 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:51,722 - stpipe.step - INFO - Step skipped.
2025-12-18 19:36:51,855 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(60, 250, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:36:52,676 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:36:52,676 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:36:52,701 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:36:52,706 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:36:52,711 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:36:52,712 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:36:55,317 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:36:55,333 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:36:55,334 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:38:17,011 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 81.67653155326843
2025-12-18 19:38:17,085 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:38:17,273 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:17,295 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:38:17,309 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:38:17,310 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:38:17,478 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:17,493 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:38:17,495 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:38:17,496 - stpipe.step - INFO - Step xply done
2025-12-18 19:38:17,498 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:38:17,668 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:17,700 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:38:17,714 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:38:17,715 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:38:17,883 - stpipe.step - INFO - Step xply running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:17,919 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:38:17,921 - stpipe.step - INFO - Step xply done
2025-12-18 19:38:17,923 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:38:18,046 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs008/stage1/jw01386008001_04101_00001_mirimage_rateints.fits
2025-12-18 19:38:18,047 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:38:18,048 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:38:18,104 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs008/stage1/jw01386008001_04101_00001_mirimage_rate.fits
2025-12-18 19:38:18,104 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:38:18,105 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:38:18,131 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:38:18,146 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:38:18,157 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:38:18,168 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:38:18,184 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:38:18,185 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:38:18,186 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:38:18,187 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:38:18,188 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:38:18,188 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:38:18,189 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:38:18,190 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:38:18,191 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:38:18,191 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:38:18,192 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:38:18,193 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:38:18,193 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:38:18,194 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:38:18,195 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:38:18,196 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:38:18,197 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:38:18,198 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:38:18,199 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:38:18,200 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:38:18,201 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:38:18,371 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs009/uncal/jw01386009001_04101_00001_mirimage_uncal.fits'),).
2025-12-18 19:38:18,392 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs009/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:38:18,418 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386009001_04101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:38:18,421 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:38:18,422 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:38:18,422 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:38:18,423 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:38:18,424 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:38:18,424 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:38:18,424 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:38:18,425 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:38:18,426 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:38:18,426 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:38:18,426 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:38:18,427 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:38:18,427 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:38:18,428 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:38:23,007 - stpipe.step - INFO - Step group_scale running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:23,839 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:38:23,840 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:38:23,842 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:38:24,034 - stpipe.step - INFO - Step dq_init running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:24,044 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:38:24,883 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:38:24,884 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:38:24,896 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:38:24,899 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:38:25,777 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:38:25,969 - stpipe.step - INFO - Step emicorr running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:38:26,797 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:38:26,814 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:38:26,815 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:38:26,815 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:38:26,816 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:38:26,816 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:39:40,861 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:39:41,050 - stpipe.step - INFO - Step saturation running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:41,899 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:39:41,900 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:39:41,923 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:41,924 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:41,933 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:41,936 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:39:41,943 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:39:49,305 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:39:49,555 - stcal.saturation.saturation - INFO - Detected 7 A/D floor pixels
2025-12-18 19:39:49,559 - stpipe.step - INFO - Step saturation done
2025-12-18 19:39:49,750 - stpipe.step - INFO - Step ipc running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:49,751 - stpipe.step - INFO - Step skipped.
2025-12-18 19:39:49,920 - stpipe.step - INFO - Step firstframe running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:50,763 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:39:50,765 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:39:50,944 - stpipe.step - INFO - Step lastframe running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:51,801 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:39:51,989 - stpipe.step - INFO - Step reset running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:52,827 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:39:52,848 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:52,849 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:52,852 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:52,932 - stpipe.step - INFO - Step reset done
2025-12-18 19:39:53,111 - stpipe.step - INFO - Step linearity running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:39:53,941 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:39:53,967 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:53,968 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:53,977 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:39:59,661 - stpipe.step - INFO - Step linearity done
2025-12-18 19:39:59,847 - stpipe.step - INFO - Step rscd running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:00,689 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:40:00,721 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:40:00,726 - stpipe.step - INFO - Step rscd done
2025-12-18 19:40:00,916 - stpipe.step - INFO - Step dark_current running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:00,926 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:40:01,912 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:40:01,912 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:40:01,916 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:40:01,918 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:40:01,918 - stcal.dark_current.dark_sub - INFO - Science data nints=60, ngroups=250, nframes=1, groupgap=0
2025-12-18 19:40:01,919 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:40:03,524 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:40:03,693 - stpipe.step - INFO - Step refpix running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:03,694 - stpipe.step - INFO - Step skipped.
2025-12-18 19:40:03,831 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:03,832 - stpipe.step - INFO - Step skipped.
2025-12-18 19:40:03,975 - stpipe.step - INFO - Step jump running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:04,814 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:40:04,815 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:40:04,818 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:40:04,820 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:40:04,845 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:40:04,850 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:40:06,076 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:40:06,077 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:40:34,106 - stcal.jump.jump - INFO - Total elapsed time = 28.0288 sec
2025-12-18 19:40:34,293 - jwst.jump.jump_step - INFO - The execution time in seconds: 30.309790
2025-12-18 19:40:34,296 - stpipe.step - INFO - Step jump done
2025-12-18 19:40:34,481 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:34,483 - stpipe.step - INFO - Step skipped.
2025-12-18 19:40:34,654 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(60, 250, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:40:35,468 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:40:35,469 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:40:35,494 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:40:35,499 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:40:35,504 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:40:35,505 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:40:38,181 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:40:38,197 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:40:38,199 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:42:00,570 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 82.36970281600952
2025-12-18 19:42:00,639 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:42:00,817 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:00,834 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:00,848 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:42:00,849 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:42:01,006 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:01,022 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:42:01,023 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:42:01,024 - stpipe.step - INFO - Step xply done
2025-12-18 19:42:01,026 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:42:01,178 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:01,207 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:01,222 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:42:01,223 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:42:01,391 - stpipe.step - INFO - Step xply running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:01,425 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:42:01,427 - stpipe.step - INFO - Step xply done
2025-12-18 19:42:01,429 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:42:01,547 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs009/stage1/jw01386009001_04101_00001_mirimage_rateints.fits
2025-12-18 19:42:01,547 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:42:01,548 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:42:01,601 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs009/stage1/jw01386009001_04101_00001_mirimage_rate.fits
2025-12-18 19:42:01,602 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:42:01,603 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
Calibrating PSF Reference Target Files#
Look for input PSF Reference Target files. For the demo example there should be 9 files in total, one for each exposure of the PSF reference target taken in the 9-point dither pattern.
# Now let's look for input files of the form *uncal.fits from the background
# observations
sstring = os.path.join(uncal_ref_targ_dir, 'jw*mirimage*uncal.fits')
uncal_ref_targ_files = sorted(glob.glob(sstring))
# Check that these are the band/channel to use
uncal_ref_targ_files = select_mask_filter_files(uncal_ref_targ_files, use_mask, use_filter)
print('Found ' + str(len(uncal_ref_targ_files)) + ' PSF reference input files')
Found 9 PSF reference input files
Runs calwebb_detector1 module on the reference target files using the same custom parameter dictionary.
# Run the pipeline on these input files by a simple loop over files using
# our custom parameter dictionary
if dodet1:
for file in uncal_ref_targ_files:
print(file)
Detector1Pipeline.call(file, steps=det1dict, save_results=True, output_dir=det1_ref_targ_dir)
else:
print('Skipping Detector1 processing for PSF reference data')
2025-12-18 19:42:01,734 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:42:01,749 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:42:01,760 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:42:01,771 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:42:01,787 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:42:01,788 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:42:01,788 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:42:01,789 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:42:01,790 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:42:01,791 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:42:01,792 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:42:01,793 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:42:01,793 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:42:01,794 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:42:01,795 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:42:01,795 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:42:01,796 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:42:01,797 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:42:01,798 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:42:01,799 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:42:01,800 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:42:01,801 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:42:01,802 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:42:01,803 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:42:01,803 - stpipe.step - INFO - XplyStep instance created.
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00001_mirimage_uncal.fits
2025-12-18 19:42:01,967 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00001_mirimage_uncal.fits'),).
2025-12-18 19:42:01,988 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:42:02,014 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:42:02,017 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:42:02,017 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:42:02,018 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:42:02,019 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:42:02,019 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:42:02,020 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:42:02,020 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:42:02,020 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:42:02,021 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:42:02,022 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:42:02,022 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:42:02,023 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:42:02,023 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:42:02,023 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:42:02,666 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:02,790 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:42:02,791 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:42:02,793 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:42:02,972 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:02,982 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:42:03,109 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:03,110 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:03,122 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:03,125 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:42:03,247 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:42:03,403 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:03,530 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:42:03,547 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:42:03,548 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:42:03,549 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:42:03,549 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:42:03,550 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:42:15,980 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:42:16,153 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:16,264 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:42:16,265 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:42:16,287 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:16,288 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:16,298 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:16,301 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:42:16,308 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:42:17,242 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:42:17,273 - stcal.saturation.saturation - INFO - Detected 3 A/D floor pixels
2025-12-18 19:42:17,277 - stpipe.step - INFO - Step saturation done
2025-12-18 19:42:17,457 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:17,458 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:17,634 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:17,762 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:42:17,765 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:42:17,947 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:18,066 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:42:18,257 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:18,387 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:42:18,411 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:18,412 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:18,414 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:18,445 - stpipe.step - INFO - Step reset done
2025-12-18 19:42:18,628 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:18,745 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:42:18,772 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:18,773 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:18,783 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:19,544 - stpipe.step - INFO - Step linearity done
2025-12-18 19:42:19,730 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:19,864 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:42:19,897 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:42:19,901 - stpipe.step - INFO - Step rscd done
2025-12-18 19:42:20,085 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:20,095 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:42:20,362 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:20,363 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:20,366 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:20,368 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:42:20,369 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:42:20,370 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:42:20,671 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:42:20,873 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:20,874 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:21,072 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:21,073 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:21,264 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:21,394 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:42:21,394 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:42:21,397 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:21,400 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:42:21,426 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:42:21,432 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:42:21,602 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:42:21,603 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:42:25,992 - stcal.jump.jump - INFO - Total elapsed time = 4.38888 sec
2025-12-18 19:42:26,019 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.747346
2025-12-18 19:42:26,022 - stpipe.step - INFO - Step jump done
2025-12-18 19:42:26,200 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:26,201 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:26,380 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:26,496 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:42:26,497 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:26,523 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:42:26,528 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:42:26,534 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:42:26,535 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:42:26,851 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:42:26,857 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:42:26,858 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:42:34,939 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.079957008361816
2025-12-18 19:42:34,989 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:42:35,165 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:35,182 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:35,197 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:42:35,197 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:42:35,373 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:35,389 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:42:35,391 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:42:35,392 - stpipe.step - INFO - Step xply done
2025-12-18 19:42:35,394 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:42:35,572 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:35,593 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:35,608 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:42:35,609 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:42:35,781 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_uncal.fits>,).
2025-12-18 19:42:35,801 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:42:35,803 - stpipe.step - INFO - Step xply done
2025-12-18 19:42:35,805 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:42:35,880 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00001_mirimage_rateints.fits
2025-12-18 19:42:35,881 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:42:35,882 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:42:35,933 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00001_mirimage_rate.fits
2025-12-18 19:42:35,934 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:42:35,934 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:42:35,959 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:42:35,974 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:42:35,984 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:42:35,995 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:42:36,011 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:42:36,012 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:42:36,013 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:42:36,014 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:42:36,015 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:42:36,016 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:42:36,016 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:42:36,017 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:42:36,018 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:42:36,019 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:42:36,019 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:42:36,020 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:42:36,021 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:42:36,022 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:42:36,023 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:42:36,024 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:42:36,025 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:42:36,026 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:42:36,027 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:42:36,028 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:42:36,028 - stpipe.step - INFO - XplyStep instance created.
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00002_mirimage_uncal.fits
2025-12-18 19:42:36,202 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00002_mirimage_uncal.fits'),).
2025-12-18 19:42:36,223 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:42:36,250 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00002_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:42:36,253 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:42:36,254 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:42:36,255 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:42:36,255 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:42:36,256 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:42:36,257 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:42:36,257 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:42:36,258 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:42:36,259 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:42:36,259 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:42:36,260 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:42:36,261 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:42:36,261 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:42:36,262 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:42:36,940 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:37,067 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:42:37,068 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:42:37,070 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:42:37,256 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:37,266 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:42:37,399 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:37,400 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:37,413 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:37,417 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:42:37,545 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:42:37,733 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:37,860 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:42:37,877 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:42:37,878 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:42:37,879 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:42:37,880 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:42:37,880 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:42:50,520 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:42:50,698 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:50,814 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:42:50,815 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:42:50,838 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:50,839 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:50,849 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:50,853 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:42:50,860 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:42:51,789 - stcal.saturation.saturation - INFO - Detected 663 saturated pixels
2025-12-18 19:42:51,821 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:42:51,825 - stpipe.step - INFO - Step saturation done
2025-12-18 19:42:52,005 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:52,006 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:52,181 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:52,335 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:42:52,338 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:42:52,549 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:52,661 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:42:52,836 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:52,957 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:42:52,980 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:52,981 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:52,984 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:53,013 - stpipe.step - INFO - Step reset done
2025-12-18 19:42:53,190 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:53,303 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:42:53,331 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:53,332 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:53,342 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:54,066 - stpipe.step - INFO - Step linearity done
2025-12-18 19:42:54,247 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:54,370 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:42:54,403 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:42:54,406 - stpipe.step - INFO - Step rscd done
2025-12-18 19:42:54,580 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:54,590 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:42:54,841 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:54,842 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:54,846 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:42:54,848 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:42:54,849 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:42:54,849 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:42:55,140 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:42:55,321 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:55,322 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:55,502 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:55,503 - stpipe.step - INFO - Step skipped.
2025-12-18 19:42:55,673 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:42:55,795 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:42:55,796 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:42:55,799 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:42:55,802 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:42:55,827 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:42:55,832 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:42:55,999 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:42:55,999 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:43:00,493 - stcal.jump.jump - INFO - Total elapsed time = 4.49343 sec
2025-12-18 19:43:00,520 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.838470
2025-12-18 19:43:00,522 - stpipe.step - INFO - Step jump done
2025-12-18 19:43:00,699 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:00,700 - stpipe.step - INFO - Step skipped.
2025-12-18 19:43:00,874 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:00,991 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:43:00,992 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:01,018 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:43:01,023 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:43:01,028 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:43:01,029 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:43:01,389 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:43:01,394 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:43:01,395 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:43:09,418 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.021596431732178
2025-12-18 19:43:09,466 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:43:09,647 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:09,664 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:09,678 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:43:09,679 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:43:09,859 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:09,875 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:43:09,876 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:43:09,878 - stpipe.step - INFO - Step xply done
2025-12-18 19:43:09,880 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:43:10,049 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:10,070 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:10,085 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:43:10,085 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:43:10,263 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_uncal.fits>,).
2025-12-18 19:43:10,282 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:43:10,284 - stpipe.step - INFO - Step xply done
2025-12-18 19:43:10,286 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:43:10,359 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00002_mirimage_rateints.fits
2025-12-18 19:43:10,360 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:43:10,361 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:43:10,411 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00002_mirimage_rate.fits
2025-12-18 19:43:10,412 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:43:10,413 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:43:10,437 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:43:10,452 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:43:10,462 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:43:10,474 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:43:10,490 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:43:10,491 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:43:10,492 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:43:10,493 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:43:10,494 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:43:10,494 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:43:10,495 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:43:10,496 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:43:10,496 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:43:10,497 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:43:10,498 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:43:10,498 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:43:10,499 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:43:10,500 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:43:10,501 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:43:10,502 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:43:10,503 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:43:10,504 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:43:10,505 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:43:10,506 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:43:10,506 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:43:10,682 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00003_mirimage_uncal.fits'),).
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00003_mirimage_uncal.fits
2025-12-18 19:43:10,703 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:43:10,730 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00003_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:43:10,733 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:43:10,733 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:43:10,734 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:43:10,734 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:43:10,735 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:43:10,736 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:43:10,736 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:43:10,736 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:43:10,737 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:43:10,737 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:43:10,738 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:43:10,738 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:43:10,739 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:43:10,739 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:43:11,378 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:11,502 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:43:11,503 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:43:11,505 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:43:11,681 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:11,691 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:43:11,818 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:11,819 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:11,831 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:11,834 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:43:11,958 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:43:12,133 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:12,256 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:43:12,274 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:43:12,274 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:43:12,275 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:43:12,276 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:43:12,276 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:43:24,897 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:43:25,058 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:25,172 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:43:25,173 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:43:25,196 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:25,197 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:25,206 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:25,209 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:43:25,216 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:43:26,150 - stcal.saturation.saturation - INFO - Detected 660 saturated pixels
2025-12-18 19:43:26,181 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:43:26,185 - stpipe.step - INFO - Step saturation done
2025-12-18 19:43:26,347 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:26,348 - stpipe.step - INFO - Step skipped.
2025-12-18 19:43:26,496 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:26,622 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:43:26,624 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:43:26,802 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:26,914 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:43:27,093 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:27,215 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:43:27,238 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:27,239 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:27,242 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:27,271 - stpipe.step - INFO - Step reset done
2025-12-18 19:43:27,443 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:27,554 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:43:27,581 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:27,582 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:27,592 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:28,332 - stpipe.step - INFO - Step linearity done
2025-12-18 19:43:28,510 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:28,636 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:43:28,669 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:43:28,672 - stpipe.step - INFO - Step rscd done
2025-12-18 19:43:28,842 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:28,851 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:43:29,097 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:29,098 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:29,101 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:29,103 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:43:29,104 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:43:29,104 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:43:29,393 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:43:29,565 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:29,566 - stpipe.step - INFO - Step skipped.
2025-12-18 19:43:29,737 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:29,738 - stpipe.step - INFO - Step skipped.
2025-12-18 19:43:29,912 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:30,033 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:43:30,034 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:43:30,037 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:30,039 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:43:30,064 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:43:30,069 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:43:30,235 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:43:30,236 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:43:34,691 - stcal.jump.jump - INFO - Total elapsed time = 4.45436 sec
2025-12-18 19:43:34,718 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.797731
2025-12-18 19:43:34,721 - stpipe.step - INFO - Step jump done
2025-12-18 19:43:34,894 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:34,895 - stpipe.step - INFO - Step skipped.
2025-12-18 19:43:35,068 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:35,184 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:43:35,185 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:35,210 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:43:35,216 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:43:35,221 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:43:35,222 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:43:35,580 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:43:35,586 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:43:35,587 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:43:43,641 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.052839517593384
2025-12-18 19:43:43,688 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:43:43,866 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:43,883 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:43,897 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:43:43,898 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:43:44,076 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:44,092 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:43:44,094 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:43:44,095 - stpipe.step - INFO - Step xply done
2025-12-18 19:43:44,097 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:43:44,263 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:44,284 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:43:44,299 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:43:44,300 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:43:44,476 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_uncal.fits>,).
2025-12-18 19:43:44,495 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:43:44,497 - stpipe.step - INFO - Step xply done
2025-12-18 19:43:44,499 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:43:44,571 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00003_mirimage_rateints.fits
2025-12-18 19:43:44,572 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:43:44,573 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:43:44,623 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00003_mirimage_rate.fits
2025-12-18 19:43:44,624 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:43:44,624 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:43:44,648 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:43:44,662 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:43:44,673 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:43:44,684 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:43:44,700 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:43:44,701 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:43:44,701 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:43:44,702 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:43:44,703 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:43:44,704 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:43:44,704 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:43:44,705 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:43:44,706 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:43:44,707 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:43:44,707 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:43:44,708 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:43:44,709 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:43:44,710 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:43:44,711 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:43:44,711 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:43:44,713 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:43:44,714 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:43:44,715 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:43:44,715 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:43:44,717 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:43:44,897 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00004_mirimage_uncal.fits'),).
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00004_mirimage_uncal.fits
2025-12-18 19:43:44,918 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:43:44,945 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00004_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:43:44,948 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:43:44,948 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:43:44,949 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:43:44,949 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:43:44,950 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:43:44,951 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:43:44,951 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:43:44,952 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:43:44,952 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:43:44,952 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:43:44,953 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:43:44,953 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:43:44,954 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:43:44,954 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:43:45,613 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:43:45,740 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:43:45,741 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:43:45,743 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:43:45,919 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:43:45,929 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:43:46,062 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:46,063 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:46,074 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:46,077 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:43:46,200 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:43:46,377 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:43:46,503 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:43:46,521 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:43:46,522 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:43:46,522 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:43:46,523 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:43:46,523 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:43:59,026 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:43:59,192 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:43:59,310 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:43:59,311 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:43:59,334 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:59,335 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:59,345 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:43:59,348 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:43:59,355 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:44:00,304 - stcal.saturation.saturation - INFO - Detected 660 saturated pixels
2025-12-18 19:44:00,334 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:44:00,338 - stpipe.step - INFO - Step saturation done
2025-12-18 19:44:00,506 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:00,507 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:00,677 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:00,803 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:44:00,805 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:44:00,979 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:01,097 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:44:01,267 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:01,397 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:44:01,420 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:01,421 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:01,423 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:01,453 - stpipe.step - INFO - Step reset done
2025-12-18 19:44:01,640 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:01,756 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:44:01,784 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:01,785 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:01,795 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:02,532 - stpipe.step - INFO - Step linearity done
2025-12-18 19:44:02,720 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:02,847 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:44:02,880 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:44:02,883 - stpipe.step - INFO - Step rscd done
2025-12-18 19:44:03,066 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:03,076 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:44:03,327 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:03,328 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:03,331 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:03,333 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:44:03,334 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:44:03,334 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:44:03,629 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:44:03,821 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:03,822 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:04,003 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:04,004 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:04,190 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:04,316 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:44:04,317 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:44:04,320 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:04,322 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:44:04,348 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:44:04,353 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:44:04,523 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:44:04,524 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:44:08,982 - stcal.jump.jump - INFO - Total elapsed time = 4.45807 sec
2025-12-18 19:44:09,010 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.811495
2025-12-18 19:44:09,013 - stpipe.step - INFO - Step jump done
2025-12-18 19:44:09,189 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:09,190 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:09,379 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:09,496 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:44:09,497 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:09,523 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:44:09,528 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:44:09,534 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:44:09,535 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:44:09,889 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:44:09,895 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:44:09,896 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:44:17,919 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.021117925643921
2025-12-18 19:44:17,966 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:44:18,127 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:18,144 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:18,158 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:44:18,159 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:44:18,320 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:18,337 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:44:18,338 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:44:18,339 - stpipe.step - INFO - Step xply done
2025-12-18 19:44:18,341 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:44:18,489 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:18,509 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:18,523 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:44:18,523 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:44:18,672 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_uncal.fits>,).
2025-12-18 19:44:18,690 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:44:18,692 - stpipe.step - INFO - Step xply done
2025-12-18 19:44:18,693 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:44:18,765 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00004_mirimage_rateints.fits
2025-12-18 19:44:18,766 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:44:18,767 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:44:18,817 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00004_mirimage_rate.fits
2025-12-18 19:44:18,818 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:44:18,818 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:44:18,842 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:44:18,856 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:44:18,866 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:44:18,877 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:44:18,893 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:44:18,893 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:44:18,894 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:44:18,895 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:44:18,896 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:44:18,896 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:44:18,897 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:44:18,898 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:44:18,898 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:44:18,899 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:44:18,900 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:44:18,900 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:44:18,901 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:44:18,902 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:44:18,902 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:44:18,903 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:44:18,905 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:44:18,905 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:44:18,906 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:44:18,907 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:44:18,908 - stpipe.step - INFO - XplyStep instance created.
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00005_mirimage_uncal.fits
2025-12-18 19:44:19,062 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00005_mirimage_uncal.fits'),).
2025-12-18 19:44:19,083 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:44:19,108 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00005_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:44:19,112 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:44:19,112 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:44:19,113 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:44:19,114 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:44:19,114 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:44:19,115 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:44:19,115 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:44:19,115 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:44:19,116 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:44:19,116 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:44:19,117 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:44:19,117 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:44:19,117 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:44:19,118 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:44:19,742 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:19,864 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:44:19,865 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:44:19,867 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:44:20,033 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:20,043 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:44:20,171 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:20,172 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:20,183 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:20,187 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:44:20,308 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:44:20,474 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:20,598 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:44:20,615 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:44:20,616 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:44:20,616 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:44:20,617 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:44:20,617 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:44:33,199 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:44:33,368 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:33,488 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:44:33,489 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:44:33,512 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:33,513 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:33,522 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:33,526 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:44:33,533 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:44:34,492 - stcal.saturation.saturation - INFO - Detected 657 saturated pixels
2025-12-18 19:44:34,524 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:44:34,527 - stpipe.step - INFO - Step saturation done
2025-12-18 19:44:34,711 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:34,712 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:34,886 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:35,009 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:44:35,012 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:44:35,189 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:35,300 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:44:35,479 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:35,602 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:44:35,625 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:35,626 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:35,629 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:35,658 - stpipe.step - INFO - Step reset done
2025-12-18 19:44:35,825 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:35,939 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:44:35,965 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:35,966 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:35,975 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:36,701 - stpipe.step - INFO - Step linearity done
2025-12-18 19:44:36,869 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:36,994 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:44:37,026 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:44:37,029 - stpipe.step - INFO - Step rscd done
2025-12-18 19:44:37,184 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:37,194 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:44:37,439 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:37,440 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:37,443 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:37,445 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:44:37,446 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:44:37,446 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:44:37,733 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:44:37,916 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:37,917 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:38,088 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:38,090 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:38,257 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:38,389 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:44:38,390 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:44:38,393 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:38,395 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:44:38,420 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:44:38,426 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:44:38,590 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:44:38,591 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:44:42,893 - stcal.jump.jump - INFO - Total elapsed time = 4.30192 sec
2025-12-18 19:44:42,919 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.654321
2025-12-18 19:44:42,922 - stpipe.step - INFO - Step jump done
2025-12-18 19:44:43,097 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:43,098 - stpipe.step - INFO - Step skipped.
2025-12-18 19:44:43,274 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:43,388 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:44:43,388 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:43,414 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:44:43,419 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:44:43,424 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:44:43,425 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:44:43,778 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:44:43,784 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:44:43,785 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:44:51,853 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.067008256912231
2025-12-18 19:44:51,902 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:44:52,085 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:52,102 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:52,117 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:44:52,118 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:44:52,293 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:52,308 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:44:52,310 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:44:52,311 - stpipe.step - INFO - Step xply done
2025-12-18 19:44:52,313 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:44:52,488 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:52,508 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:44:52,523 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:44:52,523 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:44:52,700 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_uncal.fits>,).
2025-12-18 19:44:52,719 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:44:52,721 - stpipe.step - INFO - Step xply done
2025-12-18 19:44:52,723 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:44:52,796 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00005_mirimage_rateints.fits
2025-12-18 19:44:52,797 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:44:52,798 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:44:52,849 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00005_mirimage_rate.fits
2025-12-18 19:44:52,850 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:44:52,850 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:44:52,874 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:44:52,889 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:44:52,899 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:44:52,911 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:44:52,927 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:44:52,928 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:44:52,929 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:44:52,930 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:44:52,931 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:44:52,931 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:44:52,932 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:44:52,933 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:44:52,934 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:44:52,934 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:44:52,935 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:44:52,936 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:44:52,936 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:44:52,937 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:44:52,938 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:44:52,939 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:44:52,940 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:44:52,941 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:44:52,942 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:44:52,943 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:44:52,943 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:44:53,119 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00006_mirimage_uncal.fits'),).
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00006_mirimage_uncal.fits
2025-12-18 19:44:53,140 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:44:53,166 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00006_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:44:53,169 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:44:53,169 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:44:53,170 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:44:53,171 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:44:53,171 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:44:53,172 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:44:53,172 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:44:53,172 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:44:53,173 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:44:53,173 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:44:53,174 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:44:53,174 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:44:53,174 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:44:53,175 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:44:53,833 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:44:53,956 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:44:53,957 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:44:53,959 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:44:54,139 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:44:54,149 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:44:54,279 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:54,280 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:54,293 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:44:54,297 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:44:54,420 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:44:54,602 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:44:54,727 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:44:54,745 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:44:54,745 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:44:54,746 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:44:54,747 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:44:54,747 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:45:07,237 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:45:07,390 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:07,506 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:45:07,507 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:45:07,529 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:07,530 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:07,540 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:07,543 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:45:07,550 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:45:08,498 - stcal.saturation.saturation - INFO - Detected 645 saturated pixels
2025-12-18 19:45:08,529 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:45:08,533 - stpipe.step - INFO - Step saturation done
2025-12-18 19:45:08,692 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:08,693 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:08,866 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:09,008 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:45:09,011 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:45:09,167 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:09,278 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:45:09,432 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:09,556 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:45:09,579 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:09,580 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:09,583 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:09,612 - stpipe.step - INFO - Step reset done
2025-12-18 19:45:09,767 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:09,881 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:45:09,907 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:09,908 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:09,917 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:10,630 - stpipe.step - INFO - Step linearity done
2025-12-18 19:45:10,797 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:10,918 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:45:10,950 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:45:10,953 - stpipe.step - INFO - Step rscd done
2025-12-18 19:45:11,113 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:11,123 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:45:11,368 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:11,369 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:11,373 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:11,375 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:45:11,375 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:45:11,376 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:45:11,661 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:45:11,817 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:11,818 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:11,957 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:11,958 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:12,109 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:12,231 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:45:12,232 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:45:12,235 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:12,237 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:45:12,262 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:45:12,267 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:45:12,429 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:45:12,430 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:45:16,717 - stcal.jump.jump - INFO - Total elapsed time = 4.28693 sec
2025-12-18 19:45:16,744 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.626191
2025-12-18 19:45:16,746 - stpipe.step - INFO - Step jump done
2025-12-18 19:45:16,915 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:16,916 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:17,087 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:17,204 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:45:17,205 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:17,231 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:45:17,236 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:45:17,241 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:45:17,242 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:45:17,578 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:45:17,584 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:45:17,585 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:45:25,640 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.053704738616943
2025-12-18 19:45:25,687 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:45:25,844 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:25,861 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:25,875 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:45:25,875 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:45:26,028 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:26,045 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:45:26,046 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:45:26,047 - stpipe.step - INFO - Step xply done
2025-12-18 19:45:26,049 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:45:26,194 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:26,214 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:26,228 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:45:26,229 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:45:26,388 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_uncal.fits>,).
2025-12-18 19:45:26,407 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:45:26,409 - stpipe.step - INFO - Step xply done
2025-12-18 19:45:26,411 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:45:26,483 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00006_mirimage_rateints.fits
2025-12-18 19:45:26,484 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:45:26,485 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:45:26,535 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00006_mirimage_rate.fits
2025-12-18 19:45:26,536 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:45:26,536 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:45:26,559 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:45:26,574 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:45:26,584 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:45:26,595 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:45:26,610 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:45:26,611 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:45:26,612 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:45:26,613 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:45:26,613 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:45:26,614 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:45:26,615 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:45:26,616 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:45:26,617 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:45:26,617 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:45:26,618 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:45:26,619 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:45:26,619 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:45:26,620 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:45:26,621 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:45:26,622 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:45:26,623 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:45:26,624 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:45:26,625 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:45:26,625 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:45:26,626 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:45:26,791 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00007_mirimage_uncal.fits'),).
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00007_mirimage_uncal.fits
2025-12-18 19:45:26,811 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:45:26,838 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00007_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:45:26,841 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:45:26,842 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:45:26,843 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:45:26,843 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:45:26,844 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:45:26,844 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:45:26,844 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:45:26,845 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:45:26,845 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:45:26,846 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:45:26,847 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:45:26,847 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:45:26,847 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:45:26,848 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:45:27,532 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:27,663 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:45:27,664 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:45:27,666 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:45:27,850 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:27,860 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:45:27,993 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:27,993 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:28,005 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:28,009 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:45:28,154 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:45:28,341 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:28,469 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:45:28,486 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:45:28,487 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:45:28,488 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:45:28,489 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:45:28,489 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:45:41,231 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:45:41,417 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:41,532 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:45:41,533 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:45:41,557 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:41,558 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:41,568 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:41,572 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:45:41,579 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:45:42,537 - stcal.saturation.saturation - INFO - Detected 639 saturated pixels
2025-12-18 19:45:42,569 - stcal.saturation.saturation - INFO - Detected 1 A/D floor pixels
2025-12-18 19:45:42,573 - stpipe.step - INFO - Step saturation done
2025-12-18 19:45:42,755 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:42,756 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:42,941 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:43,067 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:45:43,069 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:45:43,255 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:43,372 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:45:43,561 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:43,687 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:45:43,710 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:43,711 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:43,714 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:43,744 - stpipe.step - INFO - Step reset done
2025-12-18 19:45:43,925 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:44,041 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:45:44,068 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:44,069 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:44,079 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:44,815 - stpipe.step - INFO - Step linearity done
2025-12-18 19:45:45,004 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:45,131 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:45:45,164 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:45:45,167 - stpipe.step - INFO - Step rscd done
2025-12-18 19:45:45,353 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:45,363 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:45:45,621 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:45,622 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:45,626 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:45:45,628 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:45:45,628 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:45:45,629 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:45:45,921 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:45:46,116 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:46,117 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:46,293 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:46,295 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:46,476 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:46,599 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:45:46,600 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:45:46,603 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:46,605 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:45:46,630 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:45:46,636 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:45:46,805 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:45:46,805 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:45:51,177 - stcal.jump.jump - INFO - Total elapsed time = 4.37179 sec
2025-12-18 19:45:51,205 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.720280
2025-12-18 19:45:51,208 - stpipe.step - INFO - Step jump done
2025-12-18 19:45:51,391 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:51,392 - stpipe.step - INFO - Step skipped.
2025-12-18 19:45:51,573 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:45:51,691 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:45:51,692 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:45:51,719 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:45:51,725 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:45:51,730 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:45:51,731 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:45:52,086 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:45:52,092 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:45:52,094 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:46:00,154 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.059316396713257
2025-12-18 19:46:00,202 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:46:00,392 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:46:00,409 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:00,424 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:46:00,425 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:46:00,610 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:46:00,626 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:46:00,627 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:46:00,628 - stpipe.step - INFO - Step xply done
2025-12-18 19:46:00,630 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:46:00,811 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:46:00,831 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:00,846 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:46:00,846 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:46:01,025 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_uncal.fits>,).
2025-12-18 19:46:01,045 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:46:01,046 - stpipe.step - INFO - Step xply done
2025-12-18 19:46:01,048 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:46:01,124 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00007_mirimage_rateints.fits
2025-12-18 19:46:01,125 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:46:01,126 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:46:01,179 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00007_mirimage_rate.fits
2025-12-18 19:46:01,180 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:46:01,180 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:46:01,206 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:46:01,222 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:46:01,232 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:46:01,244 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:46:01,260 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:46:01,261 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:46:01,262 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:46:01,263 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:46:01,263 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:46:01,264 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:46:01,265 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:46:01,266 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:46:01,267 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:46:01,267 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:46:01,268 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:46:01,269 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:46:01,270 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:46:01,270 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:46:01,271 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:46:01,272 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:46:01,274 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:46:01,275 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:46:01,275 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:46:01,276 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:46:01,277 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:46:01,461 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00008_mirimage_uncal.fits'),).
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00008_mirimage_uncal.fits
2025-12-18 19:46:01,483 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:46:01,509 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00008_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:46:01,512 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:46:01,513 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:46:01,514 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:46:01,514 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:46:01,515 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:46:01,515 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:46:01,516 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:46:01,516 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:46:01,517 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:46:01,518 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:46:01,518 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:46:01,518 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:46:01,519 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:46:01,520 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:46:02,188 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:02,317 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:46:02,318 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:46:02,320 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:46:02,510 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:02,520 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:46:02,655 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:02,656 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:02,669 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:02,672 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:46:02,819 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:46:03,005 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:03,133 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:46:03,151 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:46:03,151 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:46:03,152 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:46:03,152 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:46:03,153 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:46:15,706 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:46:15,894 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:16,008 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:46:16,009 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:46:16,032 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:16,033 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:16,044 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:16,047 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:46:16,055 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:46:17,005 - stcal.saturation.saturation - INFO - Detected 645 saturated pixels
2025-12-18 19:46:17,038 - stcal.saturation.saturation - INFO - Detected 11 A/D floor pixels
2025-12-18 19:46:17,041 - stpipe.step - INFO - Step saturation done
2025-12-18 19:46:17,226 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:17,227 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:17,407 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:17,531 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:46:17,533 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:46:17,719 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:17,839 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:46:18,025 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:18,149 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:46:18,172 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:18,173 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:18,175 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:18,206 - stpipe.step - INFO - Step reset done
2025-12-18 19:46:18,394 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:18,504 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:46:18,532 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:18,533 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:18,542 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:19,274 - stpipe.step - INFO - Step linearity done
2025-12-18 19:46:19,454 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:19,576 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:46:19,609 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:46:19,612 - stpipe.step - INFO - Step rscd done
2025-12-18 19:46:19,793 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:19,803 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:46:20,056 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:20,057 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:20,060 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:20,062 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:46:20,063 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:46:20,064 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:46:20,354 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:46:20,554 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:20,555 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:20,735 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:20,736 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:20,918 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:21,044 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:46:21,045 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:46:21,047 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:21,050 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:46:21,076 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:46:21,082 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:46:21,249 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:46:21,249 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:46:25,570 - stcal.jump.jump - INFO - Total elapsed time = 4.32066 sec
2025-12-18 19:46:25,597 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.671095
2025-12-18 19:46:25,600 - stpipe.step - INFO - Step jump done
2025-12-18 19:46:25,786 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:25,787 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:25,969 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:26,083 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:46:26,084 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:26,110 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:46:26,116 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:46:26,121 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:46:26,122 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:46:26,469 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:46:26,475 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:46:26,476 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:46:34,631 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.153657674789429
2025-12-18 19:46:34,680 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:46:34,872 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:34,889 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:34,904 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:46:34,905 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:46:35,095 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:35,111 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:46:35,112 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:46:35,114 - stpipe.step - INFO - Step xply done
2025-12-18 19:46:35,116 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:46:35,303 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:35,323 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:35,339 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:46:35,339 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:46:35,521 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_uncal.fits>,).
2025-12-18 19:46:35,540 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:46:35,542 - stpipe.step - INFO - Step xply done
2025-12-18 19:46:35,544 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:46:35,619 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00008_mirimage_rateints.fits
2025-12-18 19:46:35,620 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:46:35,621 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:46:35,673 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00008_mirimage_rate.fits
2025-12-18 19:46:35,674 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:46:35,675 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:46:35,701 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:46:35,717 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:46:35,729 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:46:35,740 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:46:35,758 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:46:35,759 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:46:35,760 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:46:35,761 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:46:35,762 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:46:35,762 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:46:35,763 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:46:35,764 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:46:35,765 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:46:35,766 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:46:35,766 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:46:35,767 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:46:35,768 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:46:35,769 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:46:35,770 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:46:35,771 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:46:35,772 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:46:35,773 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:46:35,774 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:46:35,775 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:46:35,775 - stpipe.step - INFO - XplyStep instance created.
/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00009_mirimage_uncal.fits
2025-12-18 19:46:35,965 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/uncal/jw01386007001_04101_00009_mirimage_uncal.fits'),).
2025-12-18 19:46:35,987 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:46:36,014 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00009_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:46:36,018 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:46:36,018 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:46:36,019 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:46:36,020 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:46:36,020 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:46:36,021 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:46:36,022 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:46:36,022 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:46:36,023 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:46:36,023 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:46:36,024 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:46:36,024 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:46:36,025 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:46:36,025 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:46:36,695 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:36,824 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:46:36,825 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:46:36,827 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:46:37,024 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:37,034 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:46:37,170 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:37,171 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:37,183 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:37,187 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:46:37,319 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:46:37,516 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:37,650 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:46:37,668 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:46:37,669 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:46:37,670 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:46:37,670 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:46:37,671 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:46:50,401 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:46:50,588 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:50,703 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:46:50,704 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:46:50,728 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:50,729 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:50,738 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:50,741 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:46:50,749 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:46:51,706 - stcal.saturation.saturation - INFO - Detected 648 saturated pixels
2025-12-18 19:46:51,741 - stcal.saturation.saturation - INFO - Detected 0 A/D floor pixels
2025-12-18 19:46:51,745 - stpipe.step - INFO - Step saturation done
2025-12-18 19:46:51,939 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:51,940 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:52,134 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:52,261 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:46:52,263 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:46:52,452 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:52,567 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:46:52,789 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:52,916 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:46:52,939 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:52,940 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:52,943 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:52,974 - stpipe.step - INFO - Step reset done
2025-12-18 19:46:53,159 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:53,274 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:46:53,301 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:53,302 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:53,312 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:54,043 - stpipe.step - INFO - Step linearity done
2025-12-18 19:46:54,231 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:54,359 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:46:54,393 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:46:54,397 - stpipe.step - INFO - Step rscd done
2025-12-18 19:46:54,579 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:54,589 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:46:54,844 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:54,844 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:54,848 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:46:54,850 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:46:54,851 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:46:54,851 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:46:55,147 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:46:55,345 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:55,346 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:55,533 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:55,534 - stpipe.step - INFO - Step skipped.
2025-12-18 19:46:55,720 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:46:55,848 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:46:55,849 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:46:55,851 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:46:55,854 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:46:55,880 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:46:55,886 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:46:56,052 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:46:56,052 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:47:00,436 - stcal.jump.jump - INFO - Total elapsed time = 4.38401 sec
2025-12-18 19:47:00,464 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.735811
2025-12-18 19:47:00,466 - stpipe.step - INFO - Step jump done
2025-12-18 19:47:00,658 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:00,659 - stpipe.step - INFO - Step skipped.
2025-12-18 19:47:00,845 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:00,960 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:47:00,961 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:47:00,988 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:47:00,994 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:47:01,000 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:47:01,000 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:47:01,354 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:47:01,360 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:47:01,361 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:47:09,524 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.161396980285645
2025-12-18 19:47:09,573 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:47:09,762 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:09,779 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:47:09,795 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:47:09,795 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:47:09,982 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:09,999 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:47:10,000 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:47:10,002 - stpipe.step - INFO - Step xply done
2025-12-18 19:47:10,004 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:47:10,188 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:10,208 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:47:10,222 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:47:10,223 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:47:10,413 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_uncal.fits>,).
2025-12-18 19:47:10,432 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:47:10,434 - stpipe.step - INFO - Step xply done
2025-12-18 19:47:10,436 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:47:10,511 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00009_mirimage_rateints.fits
2025-12-18 19:47:10,512 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:47:10,512 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:47:10,565 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00009_mirimage_rate.fits
2025-12-18 19:47:10,566 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:47:10,566 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
Calibrating Background Files#
Look for input background files and run calwebb_detector1 pipeline using the call method.
For the demo example there should be 4 background files in total: two exposures of the background target associated with the science target (taken in the 2-point dither) and two exposures of the background target associated with the PSF reference target (taken in the 2-point dither).
# Look for input files of the form *uncal.fits from the background
# observations
sstring1 = os.path.join(uncal_bg_sci_dir, 'jw*mirimage*uncal.fits')
sstring2 = os.path.join(uncal_bg_ref_targ_dir, 'jw*mirimage*uncal.fits')
uncal_bg_sci_files = sorted(glob.glob(sstring1))
uncal_bg_ref_targ_files = sorted(glob.glob(sstring2))
# Check that these are the filter to use
uncal_bg_sci_files = select_mask_filter_files(uncal_bg_sci_files, use_mask, use_filter)
uncal_bg_ref_targ_files = select_mask_filter_files(uncal_bg_ref_targ_files, use_mask, use_filter)
print('Found ' + str((len(uncal_bg_sci_files) + len(uncal_bg_ref_targ_files))) + ' background input files')
Found 4 background input files
# Run the pipeline on these input files by a simple loop over files using
# our custom parameter dictionary
if dodet1bg:
for file in uncal_bg_sci_files:
Detector1Pipeline.call(file, steps=det1dict, save_results=True, output_dir=det1_bg_sci_dir)
for file in uncal_bg_ref_targ_files:
Detector1Pipeline.call(file, steps=det1dict, save_results=True, output_dir=det1_bg_ref_targ_dir)
else:
print('Skipping Detector1 processing for BG data')
2025-12-18 19:47:10,633 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:47:10,648 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:47:10,659 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:47:10,670 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:47:10,687 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:47:10,688 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:47:10,689 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:47:10,690 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:47:10,691 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:47:10,691 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:47:10,692 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:47:10,693 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:47:10,694 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:47:10,695 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:47:10,696 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:47:10,696 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:47:10,697 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:47:10,698 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:47:10,699 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:47:10,700 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:47:10,701 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:47:10,702 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:47:10,703 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:47:10,706 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:47:10,706 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:47:10,893 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/uncal/jw01386030001_02101_00001_mirimage_uncal.fits'),).
2025-12-18 19:47:10,913 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs030/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:47:10,939 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386030001_02101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:47:10,943 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:47:10,943 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:47:10,944 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:47:10,945 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:47:10,945 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:47:10,946 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:47:10,947 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:47:10,947 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:47:10,948 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:47:10,949 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:47:10,949 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:47:10,950 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:47:10,950 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:47:10,951 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:47:14,987 - stpipe.step - INFO - Step group_scale running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:47:15,844 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:47:15,845 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:47:15,847 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:47:16,051 - stpipe.step - INFO - Step dq_init running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:47:16,061 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:47:16,939 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:47:16,940 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:47:16,952 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:47:16,955 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:47:17,868 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:47:18,073 - stpipe.step - INFO - Step emicorr running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:47:18,942 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:47:18,961 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:47:18,961 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:47:18,962 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:47:18,963 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:47:18,963 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:48:34,166 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:48:34,357 - stpipe.step - INFO - Step saturation running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:35,196 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:48:35,197 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:48:35,219 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:35,221 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:35,230 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:35,233 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:48:35,241 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:48:42,701 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:48:42,950 - stcal.saturation.saturation - INFO - Detected 4 A/D floor pixels
2025-12-18 19:48:42,954 - stpipe.step - INFO - Step saturation done
2025-12-18 19:48:43,157 - stpipe.step - INFO - Step ipc running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:43,158 - stpipe.step - INFO - Step skipped.
2025-12-18 19:48:43,343 - stpipe.step - INFO - Step firstframe running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:44,199 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:48:44,201 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:48:44,410 - stpipe.step - INFO - Step lastframe running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:45,282 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:48:45,491 - stpipe.step - INFO - Step reset running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:46,352 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:48:46,374 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:46,375 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:46,378 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:46,461 - stpipe.step - INFO - Step reset done
2025-12-18 19:48:46,667 - stpipe.step - INFO - Step linearity running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:47,521 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:48:47,547 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:47,548 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:47,558 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:53,237 - stpipe.step - INFO - Step linearity done
2025-12-18 19:48:53,443 - stpipe.step - INFO - Step rscd running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:54,319 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:48:54,352 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:48:54,358 - stpipe.step - INFO - Step rscd done
2025-12-18 19:48:54,569 - stpipe.step - INFO - Step dark_current running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:54,579 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:48:55,590 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:55,591 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:55,595 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:48:55,596 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:48:55,597 - stcal.dark_current.dark_sub - INFO - Science data nints=60, ngroups=250, nframes=1, groupgap=0
2025-12-18 19:48:55,598 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:48:57,270 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:48:57,474 - stpipe.step - INFO - Step refpix running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:57,475 - stpipe.step - INFO - Step skipped.
2025-12-18 19:48:57,649 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:57,650 - stpipe.step - INFO - Step skipped.
2025-12-18 19:48:57,830 - stpipe.step - INFO - Step jump running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:48:58,646 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:48:58,647 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:48:58,650 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:48:58,652 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:48:58,678 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:48:58,683 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:48:59,942 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:48:59,943 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:49:29,211 - stcal.jump.jump - INFO - Total elapsed time = 29.268 sec
2025-12-18 19:49:29,407 - jwst.jump.jump_step - INFO - The execution time in seconds: 31.569023
2025-12-18 19:49:29,410 - stpipe.step - INFO - Step jump done
2025-12-18 19:49:29,630 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:49:29,631 - stpipe.step - INFO - Step skipped.
2025-12-18 19:49:29,824 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(60, 250, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:49:30,719 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:49:30,719 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:49:30,746 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:49:30,752 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:49:30,757 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:49:30,758 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:49:33,526 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:49:33,542 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:49:33,543 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:50:57,112 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 83.56703567504883
2025-12-18 19:50:57,181 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:50:57,376 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:50:57,393 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:50:57,407 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:50:57,408 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:50:57,590 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:50:57,606 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:50:57,607 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:50:57,608 - stpipe.step - INFO - Step xply done
2025-12-18 19:50:57,610 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:50:57,794 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(60, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:50:57,824 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:50:57,838 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:50:57,839 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:50:58,019 - stpipe.step - INFO - Step xply running with args (<CubeModel(60, 224, 288) from jw01386030001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:50:58,055 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:50:58,056 - stpipe.step - INFO - Step xply done
2025-12-18 19:50:58,059 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:50:58,181 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rateints.fits
2025-12-18 19:50:58,182 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:50:58,183 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:50:58,238 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rate.fits
2025-12-18 19:50:58,239 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:50:58,239 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:50:58,267 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:50:58,282 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:50:58,292 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:50:58,304 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:50:58,320 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:50:58,321 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:50:58,322 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:50:58,323 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:50:58,324 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:50:58,325 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:50:58,325 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:50:58,327 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:50:58,328 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:50:58,329 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:50:58,329 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:50:58,330 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:50:58,331 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:50:58,332 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:50:58,332 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:50:58,334 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:50:58,335 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:50:58,336 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:50:58,337 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:50:58,338 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:50:58,339 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:50:58,524 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/uncal/jw01386030001_03101_00001_mirimage_uncal.fits'),).
2025-12-18 19:50:58,546 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs030/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:50:58,572 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386030001_03101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:50:58,575 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:50:58,575 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:50:58,576 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:50:58,577 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:50:58,577 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:50:58,578 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:50:58,578 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:50:58,579 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:50:58,579 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:50:58,580 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:50:58,580 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:50:58,581 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:50:58,581 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:50:58,582 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:51:02,967 - stpipe.step - INFO - Step group_scale running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:51:03,800 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:51:03,801 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:51:03,803 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:51:04,001 - stpipe.step - INFO - Step dq_init running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:51:04,011 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:51:04,872 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:51:04,873 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:51:04,884 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:51:04,888 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:51:05,781 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:51:05,985 - stpipe.step - INFO - Step emicorr running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:51:06,819 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:51:06,837 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:51:06,838 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:51:06,839 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:51:06,839 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:51:06,840 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:52:21,725 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:52:21,939 - stpipe.step - INFO - Step saturation running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:22,782 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:52:22,783 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:52:22,807 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:22,808 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:22,817 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:22,820 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:52:22,828 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:52:30,012 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:52:30,250 - stcal.saturation.saturation - INFO - Detected 2 A/D floor pixels
2025-12-18 19:52:30,254 - stpipe.step - INFO - Step saturation done
2025-12-18 19:52:30,431 - stpipe.step - INFO - Step ipc running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:30,432 - stpipe.step - INFO - Step skipped.
2025-12-18 19:52:30,597 - stpipe.step - INFO - Step firstframe running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:31,444 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:52:31,446 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:52:31,651 - stpipe.step - INFO - Step lastframe running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:32,528 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:52:32,723 - stpipe.step - INFO - Step reset running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:33,603 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:52:33,624 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:33,625 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:33,628 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:33,709 - stpipe.step - INFO - Step reset done
2025-12-18 19:52:33,904 - stpipe.step - INFO - Step linearity running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:34,776 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:52:34,801 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:34,802 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:34,811 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:40,433 - stpipe.step - INFO - Step linearity done
2025-12-18 19:52:40,611 - stpipe.step - INFO - Step rscd running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:41,470 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:52:41,503 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:52:41,507 - stpipe.step - INFO - Step rscd done
2025-12-18 19:52:41,683 - stpipe.step - INFO - Step dark_current running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:41,693 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:52:42,688 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:42,689 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:42,693 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:52:42,694 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:52:42,695 - stcal.dark_current.dark_sub - INFO - Science data nints=60, ngroups=250, nframes=1, groupgap=0
2025-12-18 19:52:42,696 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:52:44,342 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:52:44,543 - stpipe.step - INFO - Step refpix running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:44,544 - stpipe.step - INFO - Step skipped.
2025-12-18 19:52:44,715 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:44,716 - stpipe.step - INFO - Step skipped.
2025-12-18 19:52:44,889 - stpipe.step - INFO - Step jump running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:52:45,751 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:52:45,751 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:52:45,754 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:52:45,757 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:52:45,782 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:52:45,787 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:52:47,040 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:52:47,041 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:53:16,290 - stcal.jump.jump - INFO - Total elapsed time = 29.2488 sec
2025-12-18 19:53:16,482 - jwst.jump.jump_step - INFO - The execution time in seconds: 31.585870
2025-12-18 19:53:16,485 - stpipe.step - INFO - Step jump done
2025-12-18 19:53:16,690 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:53:16,691 - stpipe.step - INFO - Step skipped.
2025-12-18 19:53:16,869 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(60, 250, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:53:17,741 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:53:17,742 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:53:17,769 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:53:17,774 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:53:17,780 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:53:17,781 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:53:20,515 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:53:20,531 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:53:20,532 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:54:46,561 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 86.02677464485168
2025-12-18 19:54:46,632 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:54:46,845 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:46,863 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:54:46,878 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:54:46,879 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:54:47,068 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:47,085 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:54:47,086 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:54:47,088 - stpipe.step - INFO - Step xply done
2025-12-18 19:54:47,090 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:54:47,285 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(60, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:47,315 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:54:47,330 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:54:47,331 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:54:47,524 - stpipe.step - INFO - Step xply running with args (<CubeModel(60, 224, 288) from jw01386030001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:47,558 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:54:47,560 - stpipe.step - INFO - Step xply done
2025-12-18 19:54:47,562 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:54:47,684 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rateints.fits
2025-12-18 19:54:47,685 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:54:47,686 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:54:47,742 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rate.fits
2025-12-18 19:54:47,743 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:54:47,743 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:54:47,770 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:54:47,786 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:54:47,797 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:54:47,810 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:54:47,827 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:54:47,828 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:54:47,829 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:54:47,830 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:54:47,831 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:54:47,832 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:54:47,832 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:54:47,833 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:54:47,834 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:54:47,835 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:54:47,835 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:54:47,836 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:54:47,837 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:54:47,837 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:54:47,838 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:54:47,839 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:54:47,841 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:54:47,842 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:54:47,843 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:54:47,843 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:54:47,844 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:54:48,035 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/uncal/jw01386031001_02101_00001_mirimage_uncal.fits'),).
2025-12-18 19:54:48,056 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs031/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:54:48,083 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386031001_02101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:54:48,086 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:54:48,087 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:54:48,088 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:54:48,088 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:54:48,089 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:54:48,089 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:54:48,090 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:54:48,090 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:54:48,090 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:54:48,091 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:54:48,091 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:54:48,092 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:54:48,092 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:54:48,092 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:54:48,761 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:48,890 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:54:48,891 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:54:48,893 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:54:49,083 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:49,093 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:54:49,224 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:54:49,225 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:54:49,237 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:54:49,240 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:54:49,367 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:54:49,564 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:54:49,691 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:54:49,709 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:54:49,710 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:54:49,711 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:54:49,712 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:54:49,712 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:55:02,530 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:55:02,719 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:02,832 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:55:02,833 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:55:02,857 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:02,858 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:02,867 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:02,871 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:55:02,878 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:55:03,815 - stcal.saturation.saturation - INFO - Detected 672 saturated pixels
2025-12-18 19:55:03,847 - stcal.saturation.saturation - INFO - Detected 1 A/D floor pixels
2025-12-18 19:55:03,850 - stpipe.step - INFO - Step saturation done
2025-12-18 19:55:04,041 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:04,043 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:04,228 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:04,352 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:55:04,354 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:55:04,541 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:04,656 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:55:04,842 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:04,965 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:55:04,989 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:04,990 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:04,993 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:05,024 - stpipe.step - INFO - Step reset done
2025-12-18 19:55:05,209 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:05,326 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:55:05,353 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:05,354 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:05,364 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:06,078 - stpipe.step - INFO - Step linearity done
2025-12-18 19:55:06,267 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:06,390 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:55:06,424 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:55:06,427 - stpipe.step - INFO - Step rscd done
2025-12-18 19:55:06,617 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:06,627 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:55:06,881 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:06,882 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:06,886 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:06,888 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:55:06,888 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:55:06,889 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:55:07,180 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:55:07,373 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:07,374 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:07,554 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:07,556 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:07,739 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:07,865 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:55:07,866 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:55:07,869 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:07,872 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:55:07,898 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:55:07,904 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:55:08,072 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:55:08,073 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:55:12,641 - stcal.jump.jump - INFO - Total elapsed time = 4.56751 sec
2025-12-18 19:55:12,668 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.920440
2025-12-18 19:55:12,671 - stpipe.step - INFO - Step jump done
2025-12-18 19:55:12,865 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:12,866 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:13,056 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:13,177 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:55:13,178 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:13,205 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:55:13,212 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:55:13,218 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:55:13,219 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:55:13,571 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:55:13,577 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:55:13,578 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:55:21,644 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.063926696777344
2025-12-18 19:55:21,695 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:55:21,887 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:21,905 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:21,920 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:55:21,921 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:55:22,106 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:22,122 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:55:22,123 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:55:22,125 - stpipe.step - INFO - Step xply done
2025-12-18 19:55:22,127 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:55:22,311 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:22,332 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:22,347 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:55:22,348 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:55:22,534 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386031001_02101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:22,554 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:55:22,556 - stpipe.step - INFO - Step xply done
2025-12-18 19:55:22,558 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:55:22,634 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:55:22,635 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:55:22,635 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:55:22,688 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rate.fits
2025-12-18 19:55:22,689 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:55:22,689 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
2025-12-18 19:55:22,716 - stpipe.step - INFO - PARS-EMICORRSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-emicorrstep_0003.asdf
2025-12-18 19:55:22,733 - stpipe.step - INFO - PARS-DARKCURRENTSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-darkcurrentstep_0001.asdf
2025-12-18 19:55:22,745 - stpipe.step - INFO - PARS-JUMPSTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-jumpstep_0004.asdf
2025-12-18 19:55:22,758 - stpipe.pipeline - INFO - PARS-DETECTOR1PIPELINE parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-detector1pipeline_0008.asdf
2025-12-18 19:55:22,775 - stpipe.step - INFO - Detector1Pipeline instance created.
2025-12-18 19:55:22,777 - stpipe.step - INFO - GroupScaleStep instance created.
2025-12-18 19:55:22,777 - stpipe.step - INFO - DQInitStep instance created.
2025-12-18 19:55:22,779 - stpipe.step - INFO - EmiCorrStep instance created.
2025-12-18 19:55:22,779 - stpipe.step - INFO - SaturationStep instance created.
2025-12-18 19:55:22,781 - stpipe.step - INFO - IPCStep instance created.
2025-12-18 19:55:22,782 - stpipe.step - INFO - SuperBiasStep instance created.
2025-12-18 19:55:22,783 - stpipe.step - INFO - RefPixStep instance created.
2025-12-18 19:55:22,783 - stpipe.step - INFO - RscdStep instance created.
2025-12-18 19:55:22,784 - stpipe.step - INFO - FirstFrameStep instance created.
2025-12-18 19:55:22,785 - stpipe.step - INFO - LastFrameStep instance created.
2025-12-18 19:55:22,786 - stpipe.step - INFO - LinearityStep instance created.
2025-12-18 19:55:22,787 - stpipe.step - INFO - DarkCurrentStep instance created.
2025-12-18 19:55:22,787 - stpipe.step - INFO - ResetStep instance created.
2025-12-18 19:55:22,789 - stpipe.step - INFO - PersistenceStep instance created.
2025-12-18 19:55:22,789 - stpipe.step - INFO - ChargeMigrationStep instance created.
2025-12-18 19:55:22,792 - stpipe.step - INFO - JumpStep instance created.
2025-12-18 19:55:22,793 - stpipe.step - INFO - CleanFlickerNoiseStep instance created.
2025-12-18 19:55:22,794 - stpipe.step - INFO - RampFitStep instance created.
2025-12-18 19:55:22,795 - stpipe.step - INFO - GainScaleStep instance created.
2025-12-18 19:55:22,795 - stpipe.step - INFO - XplyStep instance created.
2025-12-18 19:55:22,990 - stpipe.step - INFO - Step Detector1Pipeline running with args (np.str_('/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/uncal/jw01386031001_03101_00001_mirimage_uncal.fits'),).
2025-12-18 19:55:23,012 - stpipe.step - INFO - Step Detector1Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs031/stage1
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_calibrated_ramp: False
steps:
group_scale:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dq_init:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
emicorr:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: joint
nints_to_phase: None
nbins: None
scale_reference: True
onthefly_corr_freq: None
use_n_cycles: 3
fit_ints_separately: False
user_supplied_reffile: None
save_intermediate_results: False
saturation:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
n_pix_grow_sat: 1
use_readpatt: True
ipc:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
superbias:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
refpix:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
odd_even_columns: True
use_side_ref_pixels: True
side_smoothing_length: 11
side_gain: 1.0
odd_even_rows: True
ovr_corr_mitigation_ftr: 3.0
preserve_irs2_refpix: False
irs2_mean_subtraction: False
refpix_algorithm: median
sigreject: 4.0
gaussmooth: 1.0
halfwidth: 30
rscd:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
firstframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bright_use_group1: True
lastframe:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
linearity:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_current:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
dark_output: None
average_dark_current: 1.0
reset:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
persistence:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
input_trapsfilled: ''
flag_pers_cutoff: 40.0
save_persistence: False
save_trapsfilled: True
modify_input: False
charge_migration:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
signal_threshold: 25000.0
jump:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
rejection_threshold: 4.0
three_group_rejection_threshold: 6.0
four_group_rejection_threshold: 5.0
maximum_cores: half
flag_4_neighbors: True
max_jump_to_flag_neighbors: 1000
min_jump_to_flag_neighbors: 30
after_jump_flag_dn1: 500
after_jump_flag_time1: 15
after_jump_flag_dn2: 1000
after_jump_flag_time2: 3000
expand_large_events: False
min_sat_area: 1
min_jump_area: 0
expand_factor: 0
use_ellipses: False
sat_required_snowball: False
min_sat_radius_extend: 0.0
sat_expand: 0
edge_size: 0
mask_snowball_core_next_int: True
snowball_time_masked_next_int: 4000
find_showers: False
max_shower_amplitude: 4.0
extend_snr_threshold: 3.0
extend_min_area: 50
extend_inner_radius: 1
extend_outer_radius: 2.6
extend_ellipse_expand_ratio: 1.1
time_masked_after_shower: 30
min_diffs_single_pass: 10
max_extended_radius: 200
minimum_groups: 3
minimum_sigclip_groups: 100
only_use_ints: True
clean_flicker_noise:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: True
suffix: None
search_output_file: True
input_dir: ''
autoparam: False
fit_method: median
fit_by_channel: False
background_method: median
background_box_size: None
mask_science_regions: False
apply_flat_field: False
n_sigma: 2.0
fit_histogram: False
single_mask: True
user_mask: None
save_mask: False
save_background: False
save_noise: False
ramp_fit:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
algorithm: OLS_C
int_name: ''
save_opt: False
opt_name: ''
suppress_one_group: True
firstgroup: None
lastgroup: None
maximum_cores: '1'
gain_scale:
pre_hooks: []
post_hooks:
- !!python/name:__main__.XplyStep ''
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
2025-12-18 19:55:23,039 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386031001_03101_00001_mirimage_uncal.fits' reftypes = ['dark', 'emicorr', 'gain', 'linearity', 'mask', 'persat', 'readnoise', 'reset', 'rscd', 'saturation', 'superbias', 'trapdensity', 'trappars']
2025-12-18 19:55:23,043 - stpipe.pipeline - INFO - Prefetch for DARK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits'.
2025-12-18 19:55:23,043 - stpipe.pipeline - INFO - Prefetch for EMICORR reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf'.
2025-12-18 19:55:23,044 - stpipe.pipeline - INFO - Prefetch for GAIN reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits'.
2025-12-18 19:55:23,044 - stpipe.pipeline - INFO - Prefetch for LINEARITY reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits'.
2025-12-18 19:55:23,045 - stpipe.pipeline - INFO - Prefetch for MASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits'.
2025-12-18 19:55:23,045 - stpipe.pipeline - INFO - Prefetch for PERSAT reference file is 'N/A'.
2025-12-18 19:55:23,046 - stpipe.pipeline - INFO - Prefetch for READNOISE reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits'.
2025-12-18 19:55:23,046 - stpipe.pipeline - INFO - Prefetch for RESET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits'.
2025-12-18 19:55:23,047 - stpipe.pipeline - INFO - Prefetch for RSCD reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits'.
2025-12-18 19:55:23,047 - stpipe.pipeline - INFO - Prefetch for SATURATION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits'.
2025-12-18 19:55:23,048 - stpipe.pipeline - INFO - Prefetch for SUPERBIAS reference file is 'N/A'.
2025-12-18 19:55:23,048 - stpipe.pipeline - INFO - Prefetch for TRAPDENSITY reference file is 'N/A'.
2025-12-18 19:55:23,048 - stpipe.pipeline - INFO - Prefetch for TRAPPARS reference file is 'N/A'.
2025-12-18 19:55:23,049 - jwst.pipeline.calwebb_detector1 - INFO - Starting calwebb_detector1 ...
2025-12-18 19:55:23,720 - stpipe.step - INFO - Step group_scale running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:23,845 - jwst.group_scale.group_scale_step - INFO - NFRAMES and FRMDIVSR are equal; correction not needed
2025-12-18 19:55:23,845 - jwst.group_scale.group_scale_step - INFO - Step will be skipped
2025-12-18 19:55:23,847 - stpipe.step - INFO - Step group_scale done
2025-12-18 19:55:24,046 - stpipe.step - INFO - Step dq_init running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:24,059 - jwst.dq_init.dq_init_step - INFO - Using MASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_mask_0036.fits
2025-12-18 19:55:24,192 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:24,193 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:24,205 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:24,209 - jwst.dq_init.dq_initialization - INFO - Extracting mask subarray to match science data
2025-12-18 19:55:24,368 - stpipe.step - INFO - Step dq_init done
2025-12-18 19:55:24,594 - stpipe.step - INFO - Step emicorr running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:24,726 - jwst.emicorr.emicorr_step - INFO - Using CRDS reference file: /home/runner/crds/references/jwst/miri/jwst_miri_emicorr_0003.asdf
2025-12-18 19:55:24,745 - jwst.emicorr.emicorr - INFO - Using reference file to get subarray case.
2025-12-18 19:55:24,746 - jwst.emicorr.emicorr - INFO - With configuration: Subarray=MASK1550, Read_pattern=FASTR1, Detector=MIRIMAGE
2025-12-18 19:55:24,747 - jwst.emicorr.emicorr - INFO - Will correct data for the following 2 frequencies:
2025-12-18 19:55:24,747 - jwst.emicorr.emicorr - INFO - ['Hz390', 'Hz10']
2025-12-18 19:55:24,748 - jwst.emicorr.emicorr - INFO - Running EMI fit with algorithm = 'joint'.
2025-12-18 19:55:37,532 - stpipe.step - INFO - Step emicorr done
2025-12-18 19:55:37,723 - stpipe.step - INFO - Step saturation running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:37,840 - jwst.saturation.saturation_step - INFO - Using SATURATION reference file /home/runner/crds/references/jwst/miri/jwst_miri_saturation_0034.fits
2025-12-18 19:55:37,841 - jwst.saturation.saturation_step - INFO - Using SUPERBIAS reference file N/A
2025-12-18 19:55:37,864 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:37,866 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:37,874 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:37,878 - jwst.saturation.saturation - INFO - Extracting reference file subarray to match science data
2025-12-18 19:55:37,885 - jwst.saturation.saturation - INFO - Using read_pattern with nframes 1
2025-12-18 19:55:38,841 - stcal.saturation.saturation - INFO - Detected 669 saturated pixels
2025-12-18 19:55:38,876 - stcal.saturation.saturation - INFO - Detected 2 A/D floor pixels
2025-12-18 19:55:38,880 - stpipe.step - INFO - Step saturation done
2025-12-18 19:55:39,078 - stpipe.step - INFO - Step ipc running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:39,079 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:39,271 - stpipe.step - INFO - Step firstframe running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:39,403 - jwst.firstframe.firstframe_sub - INFO - Number of usable bright pixels with first group not set to DO_NOT_USE: 0
2025-12-18 19:55:39,406 - stpipe.step - INFO - Step firstframe done
2025-12-18 19:55:39,600 - stpipe.step - INFO - Step lastframe running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:39,716 - stpipe.step - INFO - Step lastframe done
2025-12-18 19:55:39,916 - stpipe.step - INFO - Step reset running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:40,046 - jwst.reset.reset_step - INFO - Using RESET reference file /home/runner/crds/references/jwst/miri/jwst_miri_reset_0065.fits
2025-12-18 19:55:40,072 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:40,073 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:40,075 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:40,107 - stpipe.step - INFO - Step reset done
2025-12-18 19:55:40,308 - stpipe.step - INFO - Step linearity running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:40,422 - jwst.linearity.linearity_step - INFO - Using Linearity reference file /home/runner/crds/references/jwst/miri/jwst_miri_linearity_0032.fits
2025-12-18 19:55:40,450 - stdatamodels.dynamicdq - WARNING - Keyword RESERVED_4 does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:40,451 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:40,460 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:41,173 - stpipe.step - INFO - Step linearity done
2025-12-18 19:55:41,368 - stpipe.step - INFO - Step rscd running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:41,499 - jwst.rscd.rscd_step - INFO - Using RSCD reference file /home/runner/crds/references/jwst/miri/jwst_miri_rscd_0017.fits
2025-12-18 19:55:41,534 - jwst.rscd.rscd_sub - INFO - Number of groups to skip for integrations 2 and higher: 4
2025-12-18 19:55:41,538 - stpipe.step - INFO - Step rscd done
2025-12-18 19:55:41,735 - stpipe.step - INFO - Step dark_current running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:41,745 - jwst.dark_current.dark_current_step - INFO - Using DARK reference file /home/runner/crds/references/jwst/miri/jwst_miri_dark_0098.fits
2025-12-18 19:55:42,000 - stdatamodels.dynamicdq - WARNING - Keyword UNDERSAMP does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:42,001 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:42,005 - stdatamodels.dynamicdq - WARNING - Keyword UNRELIABLE_RESET does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:55:42,007 - jwst.dark_current.dark_current_step - INFO - Using Poisson noise from average dark current 1.0 e-/sec
2025-12-18 19:55:42,008 - stcal.dark_current.dark_sub - INFO - Science data nints=19, ngroups=100, nframes=1, groupgap=0
2025-12-18 19:55:42,008 - stcal.dark_current.dark_sub - INFO - Dark data nints=3, ngroups=500, nframes=1, groupgap=0
2025-12-18 19:55:42,308 - stpipe.step - INFO - Step dark_current done
2025-12-18 19:55:42,512 - stpipe.step - INFO - Step refpix running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:42,513 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:42,706 - stpipe.step - INFO - Step charge_migration running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:42,708 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:42,898 - stpipe.step - INFO - Step jump running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:43,025 - jwst.jump.jump_step - INFO - CR rejection threshold = 4 sigma
2025-12-18 19:55:43,025 - jwst.jump.jump_step - INFO - Maximum cores to use = half
2025-12-18 19:55:43,028 - jwst.jump.jump_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:43,031 - jwst.jump.jump_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:55:43,058 - jwst.jump.jump_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:55:43,064 - jwst.jump.jump_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:55:43,233 - stcal.jump.jump - INFO - Executing two-point difference method
2025-12-18 19:55:43,234 - stcal.jump.jump - INFO - Creating 4 processes for jump detection
2025-12-18 19:55:47,649 - stcal.jump.jump - INFO - Total elapsed time = 4.41509 sec
2025-12-18 19:55:47,676 - jwst.jump.jump_step - INFO - The execution time in seconds: 4.769565
2025-12-18 19:55:47,679 - stpipe.step - INFO - Step jump done
2025-12-18 19:55:47,873 - stpipe.step - INFO - Step clean_flicker_noise running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:47,874 - stpipe.step - INFO - Step skipped.
2025-12-18 19:55:48,065 - stpipe.step - INFO - Step ramp_fit running with args (<RampModel(19, 100, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:48,184 - jwst.ramp_fitting.ramp_fit_step - INFO - Using READNOISE reference file: /home/runner/crds/references/jwst/miri/jwst_miri_readnoise_0085.fits
2025-12-18 19:55:48,185 - jwst.ramp_fitting.ramp_fit_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:48,212 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting gain subarray to match science data
2025-12-18 19:55:48,217 - jwst.ramp_fitting.ramp_fit_step - INFO - Extracting readnoise subarray to match science data
2025-12-18 19:55:48,223 - jwst.ramp_fitting.ramp_fit_step - INFO - Using algorithm = OLS_C
2025-12-18 19:55:48,224 - jwst.ramp_fitting.ramp_fit_step - INFO - Using weighting = optimal
2025-12-18 19:55:48,583 - stcal.ramp_fitting.ols_fit - INFO - Number of multiprocessing slices: 1
2025-12-18 19:55:48,589 - stcal.ramp_fitting.ols_fit - INFO - Number of leading groups that are flagged as DO_NOT_USE: 1
2025-12-18 19:55:48,590 - stcal.ramp_fitting.ols_fit - INFO - MIRI dataset has all pixels in the final group flagged as DO_NOT_USE.
2025-12-18 19:55:56,702 - stcal.ramp_fitting.ols_fit - INFO - Ramp Fitting C Time: 8.110404968261719
2025-12-18 19:55:56,752 - stpipe.step - INFO - Step ramp_fit done
2025-12-18 19:55:56,949 - stpipe.step - INFO - Step gain_scale running with args (<ImageModel(224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:56,966 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:56,982 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:55:56,982 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:55:57,174 - stpipe.step - INFO - Step xply running with args (<ImageModel(224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:57,190 - py.warnings - WARNING - /tmp/ipykernel_2575/3735983842.py:14: DeprecationWarning: The Step.log attribute is deprecated and will be removed in a future release. Please use a local logger, retrieved via logging.getLogger.
self.log.info('Multiplied everything by one in custom step!')
2025-12-18 19:55:57,192 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:55:57,193 - stpipe.step - INFO - Step xply done
2025-12-18 19:55:57,195 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:55:57,386 - stpipe.step - INFO - Step gain_scale running with args (<CubeModel(19, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:57,407 - jwst.gain_scale.gain_scale_step - INFO - Using GAIN reference file: /home/runner/crds/references/jwst/miri/jwst_miri_gain_0032.fits
2025-12-18 19:55:57,422 - jwst.gain_scale.gain_scale_step - INFO - GAINFACT not found in gain reference file
2025-12-18 19:55:57,423 - jwst.gain_scale.gain_scale_step - INFO - Step will be skipped
2025-12-18 19:55:57,614 - stpipe.step - INFO - Step xply running with args (<CubeModel(19, 224, 288) from jw01386031001_03101_00001_mirimage_uncal.fits>,).
2025-12-18 19:55:57,634 - stpipe.Detector1Pipeline.gain_scale.xply - INFO - Multiplied everything by one in custom step!
2025-12-18 19:55:57,636 - stpipe.step - INFO - Step xply done
2025-12-18 19:55:57,638 - stpipe.step - INFO - Step gain_scale done
2025-12-18 19:55:57,715 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:55:57,716 - jwst.pipeline.calwebb_detector1 - INFO - ... ending calwebb_detector1
2025-12-18 19:55:57,717 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:55:57,770 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rate.fits
2025-12-18 19:55:57,771 - stpipe.step - INFO - Step Detector1Pipeline done
2025-12-18 19:55:57,772 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
# Print out the time benchmark
time1 = time.perf_counter()
print(f"Runtime so far: {time1 - time0:0.4f} seconds")
Runtime so far: 1779.1239 seconds
6.-Image2 Pipeline#
In this section we process our 3D countrate (rateints) products from
Stage 1 (calwebb_detector1) through the Image2 (calwebb_image2) pipeline
in order to produce Stage 2
data products (i.e., 3D calibrated calints and 3D background-subtracted bsubints data). These data products have units of MJy/sr.
In this pipeline processing stage, the background subtraction step is performed (if the data has a dedicated background defined), the world coordinate system (WCS) is assigned, the data is flat fielded, and a photometric calibration is applied to convert from units of countrate (ADU/s) to surface brightness (MJy/sr).
The resampling step is performed, to create resampled images of each dither position, but this is only a quick-look product. The resampling step occurs during the Coron3 stage by default. While the resampling step is done in the Image2 stage, the data quality from the Coron3 stage will be better since the bad pixels, which adversely affect both the centroids and photometry in individual images, will be mostly removed.
See https://jwst-docs.stsci.edu/jwst-science-calibration-pipeline/stages-of-jwst-data-processing/calwebb_image2
time_image2 = time.perf_counter()
# Set up a dictionary to define how the Image2 pipeline should be configured.
# Boilerplate dictionary setup
image2dict = {}
image2dict['assign_wcs'], image2dict['bkg_subtract'], image2dict['flat_field'], image2dict['photom'], image2dict['resample'] = {}, {}, {}, {}, {}
# Overrides for whether or not certain steps should be skipped (example)
#image2dict['resample']['skip'] = False
#image2dict['bkg_subtract']['skip'] = True
# Overrides for various reference files
# Files should be in the base local directory or provide full path
#image2dict['assign_wcs']['override_distortion'] = 'myfile.asdf' # Spatial distortion (ASDF file)
#image2dict['assign_wcs']['override_filteroffset'] = 'myfile.asdf' # Imager filter offsets (ASDF file)
#image2dict['assign_wcs']['override_specwcs'] = 'myfile.asdf' # Spectral distortion (ASDF file)
#image2dict['assign_wcs']['override_wavelengthrange'] = 'myfile.asdf' # Wavelength channel mapping (ASDF file)
#image2dict['flat_field']['override_flat'] = 'myfile.fits' # Pixel flatfield
#image2dict['photom']['override_photom'] = 'myfile.fits' # Photometric calibration array
# Save the combined background used for subtraction
image2dict['bkg_subtract']['save_combined_background'] = True
# Relevant step-specific arguments for background subtraction
#image2dict['bkg_subtract']['sigma'] = 3.0 # Number of standard deviations to use for sigma-clipping
#image2dict['bkg_subtract']['maxiters'] = None # Number of clipping iterations to perform when combining multiple background images. If None, will clip until convergence is achieved
# Relevant step-specific arguments for flat field
#image2dict['flat_field']['user_supplied_flat'] = 'myfile.fits' # Path to user-supplied Flat-field image
#image2dict['flat_field']['inverse'] = False # Whether to inverse the math operations used to apply the Flat-field (i.e. multiply instead of divide)
# Overrides for various reference files
# Files should be in the base local directory or provide full path
#image2dict['assign_wcs']['override_distortion'] = 'myfile.asdf' # Spatial distortion (ASDF file)
#image2dict['assign_wcs']['override_filteroffset'] = 'myfile.asdf' # Imager filter offsets (ASDF file)
#image2dict['flat_field']['override_flat'] = 'myfile.fits' # Pixel flatfield
#image2dict['photom']['override_photom'] = 'myfile.fits' # Photometric calibration array
Define a function to create association files for Stage 2. This will enable use of the background subtraction, if chosen above.
def writel2asn(onescifile, bgfiles, asnfile, prodname):
# Define the basic association of science files
asn = afl.asn_from_list([onescifile], rule=DMSLevel2bBase, product_name=prodname) # Wrap in array since input was single exposure
#Coron/filter configuration for this sci file
with fits.open(onescifile) as hdu:
hdu.verify()
hdr = hdu[0].header
this_mask, this_filter = hdr['CORONMSK'], hdr['FILTER']
# Find which background files are appropriate to this mask/filter and add to association
for file in bgfiles:
hdu.verify()
hdr = hdu[0].header
if hdr['FILTER'] == this_filter and hdr['CORONMSK'] == this_mask:
asn['products'][0]['members'].append({'expname': file, 'exptype': 'background'})
# Write the association to a json file
_, serialized = asn.dump()
with open(asnfile, 'w') as outfile:
outfile.write(serialized)
Find and sort all of the input files for the selected filter and coronagraphic mask, ensuring use of absolute paths.
The input files should be rateints.fits products and for the demo example there should be a total of 2 files corresponding to the science target; 9 files corresponding to the reference target; 2 files corresponding to the science background target and 2 files corresponding to the reference background target.
# Identify Science Files
# Roll 1
sstring = os.path.join(det1_sci_r1_dir, 'jw*mirimage*rateints.fits') # Use files from the detector1 output folder
sci_r1_files = sorted(glob.glob(sstring))
# Check that these are the mask/filter to use
sci_r1_files = select_mask_filter_files(sci_r1_files, use_mask, use_filter)
# Roll 2
sstring = os.path.join(det1_sci_r2_dir, 'jw*mirimage*rateints.fits') # Use files from the detector1 output folder
sci_r2_files = sorted(glob.glob(sstring))
sci_r2_files = select_mask_filter_files(sci_r2_files, use_mask, use_filter)
# Identify PSF Ref Target Files
sstring = os.path.join(det1_ref_targ_dir, 'jw*mirimage*rateints.fits')
ref_targ_files = sorted(glob.glob(sstring))
ref_targ_files = select_mask_filter_files(ref_targ_files, use_mask, use_filter)
# Background Files
# Sci Bkg
sstring = os.path.join(det1_bg_sci_dir, 'jw*mirimage*rateints.fits')
bg_sci_files = sorted(glob.glob(sstring))
bg_sci_files = select_mask_filter_files(bg_sci_files, use_mask, use_filter)
# Ref target Bkg
sstring = os.path.join(det1_bg_ref_targ_dir, 'jw*mirimage*rateints.fits')
bg_ref_targ_files = sorted(glob.glob(sstring))
bg_ref_targ_files = select_mask_filter_files(bg_ref_targ_files, use_mask, use_filter)
print('Found ' + str(len(sci_r1_files) + len(sci_r2_files)) + ' science files')
print('Found ' + str(len(ref_targ_files)) + ' reference files')
print('Found ' + str(len(bg_sci_files)) + ' science background files')
print('Found ' + str(len(bg_ref_targ_files)) + ' reference background files')
Found 2 science files
Found 9 reference files
Found 2 science background files
Found 2 reference background files
Step through each of the science files for both rolls. First creates the association file using relevant associated backgrounds and then runs calwebb_image2 processing.
if doimage2:
# Science Roll 1
# Generate a proper background-subtracting association file
for file in sci_r1_files:
asnfile = os.path.join(image2_sci_r1_dir, 'l2asn.json')
writel2asn(file, bg_sci_files, asnfile, 'Level2')
Image2Pipeline.call(asnfile, steps=image2dict, save_bsub=True, save_results=True, output_dir=image2_sci_r1_dir)
# Science Roll 2
# Generate a proper background-subtracting association file
for file in sci_r2_files:
asnfile = os.path.join(image2_sci_r2_dir, 'l2asn.json')
writel2asn(file, bg_sci_files, asnfile, 'Level2')
Image2Pipeline.call(asnfile, steps=image2dict, save_bsub=True, save_results=True, output_dir=image2_sci_r2_dir)
else:
print('Skipping Image2 processing for SCI data')
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:55:58,036 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf 1.0 K bytes (1 / 1 files) (0 / 1.0 K bytes)
2025-12-18 19:55:58,294 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:55:58,306 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:55:58,308 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:55:58,309 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:55:58,310 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:55:58,312 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:55:58,313 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:55:58,506 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs008/stage2/l2asn.json',).
2025-12-18 19:55:58,513 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs008/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:55:58,565 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:55:58,568 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf 12.4 K bytes (1 / 4 files) (0 / 832.9 K bytes)
2025-12-18 19:55:58,713 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf 2.6 K bytes (2 / 4 files) (12.4 K / 832.9 K bytes)
2025-12-18 19:55:58,827 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits 797.8 K bytes (3 / 4 files) (15.0 K / 832.9 K bytes)
2025-12-18 19:55:59,050 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits 20.2 K bytes (4 / 4 files) (812.8 K / 832.9 K bytes)
2025-12-18 19:55:59,244 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:55:59,244 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:55:59,245 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:55:59,245 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:55:59,246 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:55:59,246 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:55:59,247 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:55:59,247 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:55:59,248 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:55:59,249 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:55:59,249 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:55:59,250 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:55:59,250 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:55:59,250 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:55:59,251 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:55:59,251 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:55:59,251 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:55:59,252 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:55:59,252 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:55:59,253 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:55:59,253 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:55:59,254 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:55:59,254 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:55:59,255 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:55:59,262 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs008/stage1/jw01386008001_04101_00001_mirimage
2025-12-18 19:55:59,262 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs008/stage1/jw01386008001_04101_00001_mirimage_rateints.fits ...
2025-12-18 19:55:59,542 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(60, 224, 288) from ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:55:59,566 - jwst.background.asn_intake - INFO - Working on input <CubeModel(60, 224, 288) from ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_image2pipeline.fits> ...
2025-12-18 19:55:59,615 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:00,019 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:00,414 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_image2pipeline.fits
2025-12-18 19:56:00,469 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_combinedbackground.fits
2025-12-18 19:56:00,470 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_combinedbackground.fits.
2025-12-18 19:56:00,586 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_bsubints.fits
2025-12-18 19:56:00,587 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:00,783 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:00,899 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:00,966 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 201.142913176 -51.506910746 201.153451782 -51.509019981 201.157902908 -51.500668859 201.147337625 -51.498535547
2025-12-18 19:56:00,968 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 201.142913176 -51.506910746 201.153451782 -51.509019981 201.157902908 -51.500668859 201.147337625 -51.498535547
2025-12-18 19:56:00,969 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:01,009 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:01,208 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:01,250 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:01,251 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:01,252 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:01,253 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:01,255 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:01,256 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:01,256 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:01,257 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:01,358 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:01,554 - stpipe.step - INFO - Step photom running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:01,571 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:01,572 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:01,605 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:01,606 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:01,606 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:01,607 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:01,634 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:01,635 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:01,637 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:01,638 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994325
2025-12-18 19:56:01,639 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52796
2025-12-18 19:56:01,697 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:01,698 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs008/stage1/jw01386008001_04101_00001_mirimage
2025-12-18 19:56:01,699 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:01,699 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:01,882 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs008/stage2/jw01386008001_04101_00001_mirimage_calints.fits
2025-12-18 19:56:01,883 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:01,884 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:01,960 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:01,972 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:01,973 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:01,975 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:01,976 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:01,976 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:01,978 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:02,181 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs009/stage2/l2asn.json',).
2025-12-18 19:56:02,189 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs009/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:02,239 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:02,242 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:02,243 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:02,243 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:02,244 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:02,245 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:02,245 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:02,246 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:02,247 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:02,247 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:02,248 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:02,248 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:02,249 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:02,249 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:02,250 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:02,251 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:02,251 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:02,252 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:02,252 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:02,253 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:02,253 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:02,254 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:02,254 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:02,255 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:02,256 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:02,262 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs009/stage1/jw01386009001_04101_00001_mirimage
2025-12-18 19:56:02,263 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs009/stage1/jw01386009001_04101_00001_mirimage_rateints.fits ...
2025-12-18 19:56:02,541 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(60, 224, 288) from ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:02,566 - jwst.background.asn_intake - INFO - Working on input <CubeModel(60, 224, 288) from ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:02,610 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:03,013 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs030/stage1/jw01386030001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:03,408 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_image2pipeline.fits
2025-12-18 19:56:03,463 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_combinedbackground.fits
2025-12-18 19:56:03,464 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_combinedbackground.fits.
2025-12-18 19:56:03,580 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_bsubints.fits
2025-12-18 19:56:03,581 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:03,789 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:03,883 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:03,942 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 201.142396067 -51.506154892 201.152241442 -51.509304909 201.158819544 -51.501516925 201.148953781 -51.498340430
2025-12-18 19:56:03,944 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 201.142396067 -51.506154892 201.152241442 -51.509304909 201.158819544 -51.501516925 201.148953781 -51.498340430
2025-12-18 19:56:03,945 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:03,981 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:04,177 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:04,214 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:04,215 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:04,216 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:04,217 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:04,219 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:04,220 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:04,220 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:04,221 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:04,321 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:04,522 - stpipe.step - INFO - Step photom running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:04,534 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:04,535 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:04,572 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:04,572 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:04,573 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:04,574 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:04,598 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:04,599 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:04,600 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:04,602 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.99432
2025-12-18 19:56:04,602 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52798
2025-12-18 19:56:04,664 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:04,665 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs009/stage1/jw01386009001_04101_00001_mirimage
2025-12-18 19:56:04,666 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:04,666 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:04,847 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs009/stage2/jw01386009001_04101_00001_mirimage_calints.fits
2025-12-18 19:56:04,847 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:04,848 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
Step through each of the reference target files. First creates the association file using relevant associated backgrounds and then runs calwebb_image2 processing.
if doimage2:
for file in ref_targ_files:
# Extract the dither number to use in asn filename
match = re.compile(r'(\d{5})_mirimage').search(file)
# Generate a proper background-subtracting association file
asnfile = os.path.join(image2_ref_targ_dir, match.group(1) + '_l2asn.json')
writel2asn(file, bg_ref_targ_files, asnfile, 'Level2')
Image2Pipeline.call(asnfile, steps=image2dict, save_bsub=True, save_results=True, output_dir=image2_ref_targ_dir)
else:
print('Skipping Image2 processing for PSF REF target data')
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:04,930 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:04,942 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:04,943 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:04,944 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:04,945 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:04,946 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:04,947 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:05,140 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00001_l2asn.json',).
2025-12-18 19:56:05,148 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:05,200 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00001_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:05,204 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:05,205 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:05,205 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:05,206 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:05,206 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:05,207 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:05,208 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:05,209 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:05,209 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:05,210 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:05,210 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:05,211 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:05,211 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:05,212 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:05,212 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:05,213 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:05,213 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:05,214 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:05,214 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:05,215 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:05,215 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:05,217 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:05,217 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:05,218 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:05,224 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00001_mirimage
2025-12-18 19:56:05,225 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00001_mirimage_rateints.fits ...
2025-12-18 19:56:05,467 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:05,490 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:05,522 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:05,637 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:05,763 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_image2pipeline.fits
2025-12-18 19:56:05,799 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_combinedbackground.fits
2025-12-18 19:56:05,800 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_combinedbackground.fits.
2025-12-18 19:56:05,879 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_bsubints.fits
2025-12-18 19:56:05,880 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:06,078 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:06,176 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:06,232 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561515189 -42.103134107 209.570302778 -42.105362391 209.574241503 -42.097063252 209.565431703 -42.094810476
2025-12-18 19:56:06,233 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561515189 -42.103134107 209.570302778 -42.105362391 209.574241503 -42.097063252 209.565431703 -42.094810476
2025-12-18 19:56:06,234 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:06,270 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:06,462 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:06,498 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:06,499 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:06,500 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:06,501 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:06,503 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:06,503 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:06,504 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:06,505 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:06,559 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:06,751 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_bsubints.fits>,).
2025-12-18 19:56:06,764 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:06,765 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:06,796 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:06,797 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:06,797 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:06,798 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:06,822 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:06,823 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:06,824 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:06,825 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994333
2025-12-18 19:56:06,827 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52793
2025-12-18 19:56:06,849 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:06,850 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00001_mirimage
2025-12-18 19:56:06,851 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:06,851 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:06,982 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00001_mirimage_calints.fits
2025-12-18 19:56:06,983 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:06,984 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:07,061 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:07,073 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:07,074 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:07,075 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:07,076 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:07,077 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:07,078 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:07,272 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00002_l2asn.json',).
2025-12-18 19:56:07,279 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:07,332 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00002_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:07,335 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:07,336 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:07,337 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:07,337 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:07,337 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:07,338 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:07,338 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:07,339 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:07,339 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:07,340 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:07,341 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:07,341 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:07,341 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:07,342 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:07,342 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:07,343 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:07,343 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:07,343 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:07,344 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:07,344 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:07,345 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:07,345 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:07,345 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:07,346 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:07,353 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00002_mirimage
2025-12-18 19:56:07,354 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00002_mirimage_rateints.fits ...
2025-12-18 19:56:07,592 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:07,610 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:07,643 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:07,759 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:07,882 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_image2pipeline.fits
2025-12-18 19:56:07,917 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_combinedbackground.fits
2025-12-18 19:56:07,918 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_combinedbackground.fits.
2025-12-18 19:56:07,994 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_bsubints.fits
2025-12-18 19:56:07,995 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:08,185 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_bsubints.fits>,).
2025-12-18 19:56:08,280 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:08,334 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561516313 -42.103131494 209.570303902 -42.105359776 209.574242625 -42.097060637 209.565432824 -42.094807863
2025-12-18 19:56:08,336 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561516313 -42.103131494 209.570303902 -42.105359776 209.574242625 -42.097060637 209.565432824 -42.094807863
2025-12-18 19:56:08,337 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:08,372 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:08,561 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_bsubints.fits>,).
2025-12-18 19:56:08,597 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:08,598 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:08,599 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:08,600 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:08,601 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:08,602 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:08,603 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:08,603 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:08,657 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:08,854 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_bsubints.fits>,).
2025-12-18 19:56:08,867 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:08,868 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:08,900 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:08,900 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:08,901 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:08,902 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:08,927 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:08,928 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:08,930 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:08,931 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994333
2025-12-18 19:56:08,931 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52793
2025-12-18 19:56:08,955 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:08,956 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00002_mirimage
2025-12-18 19:56:08,957 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:08,957 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:09,089 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00002_mirimage_calints.fits
2025-12-18 19:56:09,090 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:09,090 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:09,168 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:09,180 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:09,181 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:09,182 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:09,183 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:09,184 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:09,186 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:09,382 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00003_l2asn.json',).
2025-12-18 19:56:09,390 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:09,441 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00003_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:09,444 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:09,445 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:09,446 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:09,446 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:09,446 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:09,447 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:09,447 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:09,448 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:09,448 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:09,449 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:09,450 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:09,450 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:09,450 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:09,451 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:09,451 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:09,451 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:09,452 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:09,452 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:09,453 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:09,453 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:09,453 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:09,454 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:09,454 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:09,455 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:09,462 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00003_mirimage
2025-12-18 19:56:09,462 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00003_mirimage_rateints.fits ...
2025-12-18 19:56:09,702 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:09,720 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:09,752 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:09,871 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:09,994 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_image2pipeline.fits
2025-12-18 19:56:10,029 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_combinedbackground.fits
2025-12-18 19:56:10,030 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_combinedbackground.fits.
2025-12-18 19:56:10,107 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_bsubints.fits
2025-12-18 19:56:10,108 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:10,304 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_bsubints.fits>,).
2025-12-18 19:56:10,400 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:10,454 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561512784 -42.103130586 209.570300372 -42.105358869 209.574239097 -42.097059730 209.565429296 -42.094806955
2025-12-18 19:56:10,455 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561512784 -42.103130586 209.570300372 -42.105358869 209.574239097 -42.097059730 209.565429296 -42.094806955
2025-12-18 19:56:10,456 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:10,491 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:10,681 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_bsubints.fits>,).
2025-12-18 19:56:10,717 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:10,718 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:10,719 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:10,720 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:10,722 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:10,722 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:10,723 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:10,723 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:10,777 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:10,973 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_bsubints.fits>,).
2025-12-18 19:56:10,985 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:10,986 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:11,018 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:11,018 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:11,019 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:11,020 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:11,044 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:11,045 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:11,046 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:11,048 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994332
2025-12-18 19:56:11,048 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52793
2025-12-18 19:56:11,071 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:11,072 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00003_mirimage
2025-12-18 19:56:11,073 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:11,073 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:11,203 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00003_mirimage_calints.fits
2025-12-18 19:56:11,204 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:11,204 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:11,277 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:11,289 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:11,290 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:11,291 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:11,292 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:11,293 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:11,294 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:11,489 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00004_l2asn.json',).
2025-12-18 19:56:11,497 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:11,546 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00004_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:11,549 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:11,550 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:11,550 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:11,551 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:11,551 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:11,551 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:11,552 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:11,552 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:11,553 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:11,553 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:11,553 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:11,554 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:11,554 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:11,555 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:11,555 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:11,555 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:11,556 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:11,556 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:11,557 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:11,557 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:11,558 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:11,558 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:11,558 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:11,559 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:11,566 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00004_mirimage
2025-12-18 19:56:11,567 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00004_mirimage_rateints.fits ...
2025-12-18 19:56:11,807 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:11,825 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:11,859 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:11,975 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:12,098 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_image2pipeline.fits
2025-12-18 19:56:12,133 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_combinedbackground.fits
2025-12-18 19:56:12,134 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_combinedbackground.fits.
2025-12-18 19:56:12,211 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_bsubints.fits
2025-12-18 19:56:12,212 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:12,407 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_bsubints.fits>,).
2025-12-18 19:56:12,501 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:12,555 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561511547 -42.103133207 209.570299136 -42.105361490 209.574237860 -42.097062351 209.565428060 -42.094809576
2025-12-18 19:56:12,557 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561511547 -42.103133207 209.570299136 -42.105361490 209.574237860 -42.097062351 209.565428060 -42.094809576
2025-12-18 19:56:12,558 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:12,593 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:12,789 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_bsubints.fits>,).
2025-12-18 19:56:12,826 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:12,827 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:12,827 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:12,829 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:12,830 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:12,831 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:12,831 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:12,832 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:12,887 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:13,084 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_bsubints.fits>,).
2025-12-18 19:56:13,097 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:13,098 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:13,130 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:13,130 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:13,131 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:13,131 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:13,157 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:13,157 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:13,159 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:13,160 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994332
2025-12-18 19:56:13,160 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52793
2025-12-18 19:56:13,182 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:13,183 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00004_mirimage
2025-12-18 19:56:13,185 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:13,185 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:13,315 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00004_mirimage_calints.fits
2025-12-18 19:56:13,316 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:13,316 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:13,390 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:13,401 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:13,403 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:13,404 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:13,405 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:13,405 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:13,407 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:13,604 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00005_l2asn.json',).
2025-12-18 19:56:13,613 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:13,664 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00005_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:13,667 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:13,668 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:13,668 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:13,669 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:13,669 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:13,669 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:13,670 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:13,671 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:13,671 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:13,672 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:13,672 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:13,673 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:13,673 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:13,673 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:13,674 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:13,674 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:13,674 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:13,675 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:13,675 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:13,676 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:13,676 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:13,677 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:13,677 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:13,677 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:13,684 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00005_mirimage
2025-12-18 19:56:13,685 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00005_mirimage_rateints.fits ...
2025-12-18 19:56:13,931 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:13,949 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:13,982 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:14,101 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:14,224 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_image2pipeline.fits
2025-12-18 19:56:14,260 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_combinedbackground.fits
2025-12-18 19:56:14,261 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_combinedbackground.fits.
2025-12-18 19:56:14,337 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_bsubints.fits
2025-12-18 19:56:14,338 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:14,533 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_bsubints.fits>,).
2025-12-18 19:56:14,628 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:14,683 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561510304 -42.103135829 209.570297894 -42.105364112 209.574236618 -42.097064973 209.565426817 -42.094812198
2025-12-18 19:56:14,684 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561510304 -42.103135829 209.570297894 -42.105364112 209.574236618 -42.097064973 209.565426817 -42.094812198
2025-12-18 19:56:14,685 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:14,720 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:14,913 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_bsubints.fits>,).
2025-12-18 19:56:14,949 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:14,950 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:14,951 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:14,953 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:14,954 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:14,955 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:14,955 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:14,956 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:15,010 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:15,209 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_bsubints.fits>,).
2025-12-18 19:56:15,222 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:15,223 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:15,255 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:15,256 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:15,256 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:15,257 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:15,282 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:15,283 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:15,284 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:15,285 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994332
2025-12-18 19:56:15,286 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52793
2025-12-18 19:56:15,308 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:15,309 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00005_mirimage
2025-12-18 19:56:15,310 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:15,311 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:15,440 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00005_mirimage_calints.fits
2025-12-18 19:56:15,440 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:15,441 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:15,514 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:15,526 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:15,527 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:15,528 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:15,529 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:15,530 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:15,532 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:15,729 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00006_l2asn.json',).
2025-12-18 19:56:15,737 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:15,787 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00006_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:15,791 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:15,791 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:15,792 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:15,792 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:15,793 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:15,793 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:15,793 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:15,794 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:15,794 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:15,795 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:15,796 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:15,796 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:15,797 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:15,797 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:15,797 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:15,798 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:15,798 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:15,799 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:15,799 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:15,800 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:15,800 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:15,801 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:15,801 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:15,802 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:15,808 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00006_mirimage
2025-12-18 19:56:15,809 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00006_mirimage_rateints.fits ...
2025-12-18 19:56:16,053 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:16,071 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:16,103 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:16,221 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:16,345 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_image2pipeline.fits
2025-12-18 19:56:16,382 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_combinedbackground.fits
2025-12-18 19:56:16,383 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_combinedbackground.fits.
2025-12-18 19:56:16,459 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_bsubints.fits
2025-12-18 19:56:16,460 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:16,666 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_bsubints.fits>,).
2025-12-18 19:56:16,764 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:16,819 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561513845 -42.103136738 209.570301434 -42.105365021 209.574240158 -42.097065882 209.565430357 -42.094813107
2025-12-18 19:56:16,821 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561513845 -42.103136738 209.570301434 -42.105365021 209.574240158 -42.097065882 209.565430357 -42.094813107
2025-12-18 19:56:16,822 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:16,858 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:17,055 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_bsubints.fits>,).
2025-12-18 19:56:17,091 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:17,092 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:17,093 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:17,094 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:17,096 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:17,096 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:17,097 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:17,098 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:17,152 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:17,349 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_bsubints.fits>,).
2025-12-18 19:56:17,361 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:17,362 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:17,393 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:17,394 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:17,395 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:17,395 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:17,420 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:17,421 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:17,422 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:17,423 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994331
2025-12-18 19:56:17,424 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52794
2025-12-18 19:56:17,446 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:17,447 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00006_mirimage
2025-12-18 19:56:17,448 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:17,448 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:17,578 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00006_mirimage_calints.fits
2025-12-18 19:56:17,578 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:17,579 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:17,653 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:17,664 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:17,665 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:17,666 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:17,667 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:17,668 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:17,670 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:17,870 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00007_l2asn.json',).
2025-12-18 19:56:17,878 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:17,929 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00007_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:17,933 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:17,933 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:17,934 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:17,934 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:17,935 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:17,935 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:17,935 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:17,936 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:17,937 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:17,937 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:17,938 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:17,938 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:17,938 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:17,939 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:17,939 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:17,940 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:17,940 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:17,940 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:17,941 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:17,941 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:17,942 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:17,942 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:17,942 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:17,943 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:17,950 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00007_mirimage
2025-12-18 19:56:17,951 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00007_mirimage_rateints.fits ...
2025-12-18 19:56:18,205 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:18,223 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:18,255 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:18,372 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:18,496 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_image2pipeline.fits
2025-12-18 19:56:18,531 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_combinedbackground.fits
2025-12-18 19:56:18,532 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_combinedbackground.fits.
2025-12-18 19:56:18,608 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_bsubints.fits
2025-12-18 19:56:18,609 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:18,817 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_bsubints.fits>,).
2025-12-18 19:56:18,915 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:18,971 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561517395 -42.103137670 209.570304985 -42.105365952 209.574243709 -42.097066814 209.565433908 -42.094814039
2025-12-18 19:56:18,972 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561517395 -42.103137670 209.570304985 -42.105365952 209.574243709 -42.097066814 209.565433908 -42.094814039
2025-12-18 19:56:18,973 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:19,009 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:19,215 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_bsubints.fits>,).
2025-12-18 19:56:19,251 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:19,252 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:19,252 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:19,253 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:19,255 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:19,256 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:19,256 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:19,257 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:19,313 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:19,522 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_bsubints.fits>,).
2025-12-18 19:56:19,534 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:19,535 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:19,568 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:19,568 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:19,569 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:19,570 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:19,595 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:19,596 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:19,597 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:19,598 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.994331
2025-12-18 19:56:19,599 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52794
2025-12-18 19:56:19,622 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:19,624 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00007_mirimage
2025-12-18 19:56:19,624 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:19,625 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:19,759 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00007_mirimage_calints.fits
2025-12-18 19:56:19,760 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:19,760 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:19,837 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:19,849 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:19,851 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:19,852 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:19,853 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:19,853 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:19,855 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:20,063 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00008_l2asn.json',).
2025-12-18 19:56:20,070 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:20,125 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00008_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:20,129 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:20,129 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:20,130 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:20,131 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:20,131 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:20,132 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:20,132 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:20,133 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:20,133 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:20,134 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:20,135 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:20,136 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:20,136 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:20,137 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:20,137 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:20,138 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:20,138 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:20,139 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:20,140 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:20,140 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:20,141 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:20,141 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:20,142 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:20,142 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:20,149 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00008_mirimage
2025-12-18 19:56:20,150 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00008_mirimage_rateints.fits ...
2025-12-18 19:56:20,402 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:20,420 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:20,454 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:20,571 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:20,695 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_image2pipeline.fits
2025-12-18 19:56:20,731 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_combinedbackground.fits
2025-12-18 19:56:20,732 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_combinedbackground.fits.
2025-12-18 19:56:20,810 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_bsubints.fits
2025-12-18 19:56:20,811 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:21,012 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_bsubints.fits>,).
2025-12-18 19:56:21,108 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:21,161 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561518608 -42.103135037 209.570306197 -42.105363320 209.574244921 -42.097064181 209.565435120 -42.094811406
2025-12-18 19:56:21,162 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561518608 -42.103135037 209.570306197 -42.105363320 209.574244921 -42.097064181 209.565435120 -42.094811406
2025-12-18 19:56:21,163 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:21,198 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:21,398 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_bsubints.fits>,).
2025-12-18 19:56:21,434 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:21,435 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:21,436 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:21,437 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:21,438 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:21,439 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:21,440 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:21,440 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:21,494 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:21,696 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_bsubints.fits>,).
2025-12-18 19:56:21,709 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:21,709 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:21,741 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:21,742 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:21,742 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:21,743 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:21,767 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:21,768 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:21,769 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:21,770 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.99433
2025-12-18 19:56:21,770 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52794
2025-12-18 19:56:21,793 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:21,794 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00008_mirimage
2025-12-18 19:56:21,795 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:21,796 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:21,927 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00008_mirimage_calints.fits
2025-12-18 19:56:21,928 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:21,929 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:22,006 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:22,018 - stpipe.step - INFO - Image2Pipeline instance created.
2025-12-18 19:56:22,019 - stpipe.step - INFO - BackgroundStep instance created.
2025-12-18 19:56:22,021 - stpipe.step - INFO - AssignWcsStep instance created.
2025-12-18 19:56:22,021 - stpipe.step - INFO - FlatFieldStep instance created.
2025-12-18 19:56:22,022 - stpipe.step - INFO - PhotomStep instance created.
2025-12-18 19:56:22,024 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:22,236 - stpipe.step - INFO - Step Image2Pipeline running with args ('./miri_coro_demo_data/Obs007/stage2/00009_l2asn.json',).
2025-12-18 19:56:22,244 - stpipe.step - INFO - Step Image2Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/Obs007/stage2
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_bsub: True
steps:
bkg_subtract:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
bkg_list: None
save_combined_background: True
sigma: 3.0
maxiters: None
soss_source_percentile: 35.0
soss_bkg_percentile: None
wfss_mmag_extract: None
wfss_maxiter: 5
wfss_rms_stop: 0.0
wfss_outlier_percent: 1.0
assign_wcs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
sip_approx: True
sip_max_pix_error: 0.01
sip_degree: None
sip_max_inv_pix_error: 0.01
sip_inv_degree: None
sip_npoints: 12
slit_y_low: -0.55
slit_y_high: 0.55
nrs_ifu_slice_wcs: False
flat_field:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
save_interpolated_flat: False
user_supplied_flat: None
inverse: False
photom:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
inverse: False
source_type: None
apply_time_correction: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:22,296 - stpipe.pipeline - INFO - Prefetching reference files for dataset: '00009_l2asn.json' reftypes = ['area', 'bkg', 'camera', 'collimator', 'dflat', 'disperser', 'distortion', 'fflat', 'filteroffset', 'flat', 'fore', 'fpa', 'ifufore', 'ifupost', 'ifuslicer', 'msa', 'ote', 'photom', 'regions', 'sflat', 'specwcs', 'wavelengthrange']
2025-12-18 19:56:22,300 - stpipe.pipeline - INFO - Prefetch for AREA reference file is 'N/A'.
2025-12-18 19:56:22,300 - stpipe.pipeline - INFO - Prefetch for BKG reference file is 'N/A'.
2025-12-18 19:56:22,301 - stpipe.pipeline - INFO - Prefetch for CAMERA reference file is 'N/A'.
2025-12-18 19:56:22,301 - stpipe.pipeline - INFO - Prefetch for COLLIMATOR reference file is 'N/A'.
2025-12-18 19:56:22,302 - stpipe.pipeline - INFO - Prefetch for DFLAT reference file is 'N/A'.
2025-12-18 19:56:22,302 - stpipe.pipeline - INFO - Prefetch for DISPERSER reference file is 'N/A'.
2025-12-18 19:56:22,302 - stpipe.pipeline - INFO - Prefetch for DISTORTION reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf'.
2025-12-18 19:56:22,303 - stpipe.pipeline - INFO - Prefetch for FFLAT reference file is 'N/A'.
2025-12-18 19:56:22,303 - stpipe.pipeline - INFO - Prefetch for FILTEROFFSET reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf'.
2025-12-18 19:56:22,304 - stpipe.pipeline - INFO - Prefetch for FLAT reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits'.
2025-12-18 19:56:22,305 - stpipe.pipeline - INFO - Prefetch for FORE reference file is 'N/A'.
2025-12-18 19:56:22,305 - stpipe.pipeline - INFO - Prefetch for FPA reference file is 'N/A'.
2025-12-18 19:56:22,305 - stpipe.pipeline - INFO - Prefetch for IFUFORE reference file is 'N/A'.
2025-12-18 19:56:22,306 - stpipe.pipeline - INFO - Prefetch for IFUPOST reference file is 'N/A'.
2025-12-18 19:56:22,306 - stpipe.pipeline - INFO - Prefetch for IFUSLICER reference file is 'N/A'.
2025-12-18 19:56:22,307 - stpipe.pipeline - INFO - Prefetch for MSA reference file is 'N/A'.
2025-12-18 19:56:22,307 - stpipe.pipeline - INFO - Prefetch for OTE reference file is 'N/A'.
2025-12-18 19:56:22,308 - stpipe.pipeline - INFO - Prefetch for PHOTOM reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits'.
2025-12-18 19:56:22,308 - stpipe.pipeline - INFO - Prefetch for REGIONS reference file is 'N/A'.
2025-12-18 19:56:22,309 - stpipe.pipeline - INFO - Prefetch for SFLAT reference file is 'N/A'.
2025-12-18 19:56:22,309 - stpipe.pipeline - INFO - Prefetch for SPECWCS reference file is 'N/A'.
2025-12-18 19:56:22,309 - stpipe.pipeline - INFO - Prefetch for WAVELENGTHRANGE reference file is 'N/A'.
2025-12-18 19:56:22,310 - jwst.pipeline.calwebb_image2 - INFO - Starting calwebb_image2 ...
2025-12-18 19:56:22,310 - jwst.pipeline.calwebb_image2 - WARNING - The --save_bsub parameter is deprecated and will be removed in a future release. To toggle saving background-subtracted data, use the background step's --save_results parameter instead.
2025-12-18 19:56:22,318 - jwst.pipeline.calwebb_image2 - INFO - Processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00009_mirimage
2025-12-18 19:56:22,318 - jwst.pipeline.calwebb_image2 - INFO - Working on input /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00009_mirimage_rateints.fits ...
2025-12-18 19:56:22,577 - stpipe.step - INFO - Step bkg_subtract running with args (<CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_image2pipeline.fits>, ['/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits', '/home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits']).
2025-12-18 19:56:22,594 - jwst.background.asn_intake - INFO - Working on input <CubeModel(19, 224, 288) from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_image2pipeline.fits> ...
2025-12-18 19:56:22,629 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_02101_00001_mirimage_rateints.fits
2025-12-18 19:56:22,747 - jwst.background.background_sub - INFO - Accumulate bkg from /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs031/stage1/jw01386031001_03101_00001_mirimage_rateints.fits
2025-12-18 19:56:22,873 - jwst.background.background_sub - INFO - Subtracting avg bkg from ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_image2pipeline.fits
2025-12-18 19:56:22,911 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_combinedbackground.fits
2025-12-18 19:56:22,912 - jwst.background.background_step - INFO - Combined background written to ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_combinedbackground.fits.
2025-12-18 19:56:22,992 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_bsubints.fits
2025-12-18 19:56:22,992 - stpipe.step - INFO - Step bkg_subtract done
2025-12-18 19:56:23,210 - stpipe.step - INFO - Step assign_wcs running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_bsubints.fits>,).
2025-12-18 19:56:23,306 - jwst.assign_wcs.miri - INFO - Created a MIRI mir_4qpm pipeline with references {'distortion': '/home/runner/crds/references/jwst/miri/jwst_miri_distortion_0047.asdf', 'filteroffset': '/home/runner/crds/references/jwst/miri/jwst_miri_filteroffset_0015.asdf', 'specwcs': None, 'regions': None, 'wavelengthrange': None, 'camera': None, 'collimator': None, 'disperser': None, 'fore': None, 'fpa': None, 'msa': None, 'ote': None, 'ifupost': None, 'ifufore': None, 'ifuslicer': None}
2025-12-18 19:56:23,361 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 209.561519857 -42.103132412 209.570307446 -42.105360695 209.574246169 -42.097061556 209.565436368 -42.094808781
2025-12-18 19:56:23,362 - jwst.assign_wcs.assign_wcs - INFO - assign_wcs updated S_REGION to POLYGON ICRS 209.561519857 -42.103132412 209.570307446 -42.105360695 209.574246169 -42.097061556 209.565436368 -42.094808781
2025-12-18 19:56:23,363 - jwst.assign_wcs.assign_wcs - INFO - COMPLETED assign_wcs
2025-12-18 19:56:23,398 - stpipe.step - INFO - Step assign_wcs done
2025-12-18 19:56:23,598 - stpipe.step - INFO - Step flat_field running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_bsubints.fits>,).
2025-12-18 19:56:23,635 - stdatamodels.dynamicdq - WARNING - Keyword CDP_PARTIAL_DATA does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:23,636 - stdatamodels.dynamicdq - WARNING - Keyword CDP_LOW_QUAL does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:23,637 - stdatamodels.dynamicdq - WARNING - Keyword CDP_UNRELIABLE_ERROR does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:23,638 - stdatamodels.dynamicdq - WARNING - Keyword DIFF_PATTERN does not correspond to an existing DQ mnemonic, so will be ignored
2025-12-18 19:56:23,639 - jwst.flatfield.flat_field_step - INFO - Using FLAT reference file: /home/runner/crds/references/jwst/miri/jwst_miri_flat_0768.fits
2025-12-18 19:56:23,640 - jwst.flatfield.flat_field_step - INFO - No reference found for type FFLAT
2025-12-18 19:56:23,641 - jwst.flatfield.flat_field_step - INFO - No reference found for type SFLAT
2025-12-18 19:56:23,641 - jwst.flatfield.flat_field_step - INFO - No reference found for type DFLAT
2025-12-18 19:56:23,697 - stpipe.step - INFO - Step flat_field done
2025-12-18 19:56:23,910 - stpipe.step - INFO - Step photom running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_bsubints.fits>,).
2025-12-18 19:56:23,923 - jwst.photom.photom_step - INFO - Using photom reference file: /home/runner/crds/references/jwst/miri/jwst_miri_photom_0230.fits
2025-12-18 19:56:23,924 - jwst.photom.photom_step - INFO - Using area reference file: N/A
2025-12-18 19:56:23,957 - jwst.photom.photom - INFO - Using instrument: MIRI
2025-12-18 19:56:23,957 - jwst.photom.photom - INFO - detector: MIRIMAGE
2025-12-18 19:56:23,958 - jwst.photom.photom - INFO - exp_type: MIR_4QPM
2025-12-18 19:56:23,959 - jwst.photom.photom - INFO - filter: F1550C
2025-12-18 19:56:23,983 - jwst.photom.photom - INFO - Attempting to obtain PIXAR_SR and PIXAR_A2 values from PHOTOM reference file.
2025-12-18 19:56:23,984 - jwst.photom.photom - INFO - Values for PIXAR_SR and PIXAR_A2 obtained from PHOTOM reference file.
2025-12-18 19:56:23,986 - jwst.photom.photom - INFO - subarray: MASK1550
2025-12-18 19:56:23,987 - jwst.photom.photom - INFO - Multiplicative time dependence correction is 0.99433
2025-12-18 19:56:23,987 - jwst.photom.photom - INFO - PHOTMJSR value: 3.52794
2025-12-18 19:56:24,010 - stpipe.step - INFO - Step photom done
2025-12-18 19:56:24,011 - jwst.pipeline.calwebb_image2 - INFO - Finished processing product /home/runner/work/jwst-pipeline-notebooks/jwst-pipeline-notebooks/notebooks/MIRI/Coronagraphy/miri_coro_demo_data/Obs007/stage1/jw01386007001_04101_00009_mirimage
2025-12-18 19:56:24,012 - jwst.pipeline.calwebb_image2 - INFO - ... ending calwebb_image2
2025-12-18 19:56:24,012 - jwst.stpipe.core - INFO - Results used CRDS context: jwst_1464.pmap
2025-12-18 19:56:24,147 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/Obs007/stage2/jw01386007001_04101_00009_mirimage_calints.fits
2025-12-18 19:56:24,147 - stpipe.step - INFO - Step Image2Pipeline done
2025-12-18 19:56:24,148 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
# Print out the time benchmark
time1 = time.perf_counter()
print(f"Runtime so far: {time1 - time0:0.4f} seconds")
print(f"Runtime for Image2: {time1 - time_image2} seconds")
Runtime so far: 1805.4991 seconds
Runtime for Image2: 26.371077170000035 seconds
7.-Coron3 Pipeline#
In this section, we’ll run the Coron3 (calwebb_coron3) pipeline on the calibrated MIRI coronagraphic exposures to produce PSF-subtracted, resampled, combined images of the source object. The input to calwebb_coron3 must be in the form of an association file that lists one or more exposures of a science target and one or more reference PSF targets. The individual target and reference PSF exposures should be in the form of 3D photometrically calibrated (_calints) products from calwebb_image2 processing. Each pipeline step will loop over the 3D stack of per-integration images contained in each exposure. The relevant steps are:
outlier_detection: CR-flag all PSF and science target exposures
stack_refs: Reference PSF stacking
align_refs: Reference PSF alignment
klip: PSF subtraction with the KLIP algorithm
resample: Image resampling and World Coordinate System registration
See https://jwst-docs.stsci.edu/jwst-science-calibration-pipeline/stages-of-jwst-data-processing/calwebb_coron3
time_coron3 = time.perf_counter()
# Set up a dictionary to define how the Coron3 pipeline should be configured
# Boilerplate dictionary setup
coron3dict = {}
coron3dict['outlier_detection'], coron3dict['stack_refs'], coron3dict['align_refs'] = {}, {}, {}
coron3dict['klip'], coron3dict['resample'] = {}, {}
# Set the maximum number of KL transform rows to keep when computing the PSF fit to the target.
coron3dict['klip']['truncate'] = 25 # The maximum number of KL modes to use.
# Overrides for various reference files
# Files should be in the base local directory or provide full path
#coron3dict['align_refs']['override_psfmask'] = 'myfile.fits' # The PSFMASK reference file
# Options for adjusting performance for the outlier detection step
#coron3dict['outlier_detection']['kernel_size'] = '7 7' # Dial this to adjust the detector kernel size
#coron3dict['outlier_detection']['threshold_percent'] = 99.8 # Dial this to be more/less aggressive in outlier flagging (values closer to 100% are less aggressive)
# Options for adjusting the resample step
#coron3dict['resample']['pixfrac'] = 1.0 # Fraction by which input pixels are “shrunk” before being drizzled onto the output image grid
#coron3dict['resample']['kernel'] = 'square' # Kernel form used to distribute flux onto the output image
#coron3dict['resample']['fillval'] = 'INDEF' # Value to assign to output pixels that have zero weight or do not receive any flux from any input pixels during drizzling
#coron3dict['resample']['weight_type'] = 'ivm' # Weighting type for each input image.
#coron3dict['resample']['output_shape'] = None
#coron3dict['resample']['crpix'] = None
#coron3dict['resample']['crval'] = None
#coron3dict['resample']['rotation'] = None
#coron3dict['resample']['pixel_scale_ratio'] = 1.0
#coron3dict['resample']['pixel_scale'] = None
#coron3dict['resample']['output_wcs'] = ''
#coron3dict['resample']['single'] = False
#coron3dict['resample']['blendheaders'] = True
Define a function to create association files for Stage 3. It creates an association from a list of science exposures and a list of PSF reference exposures.
def writel3asn(scifiles, ref_targ_files, asnfile, prodname):
"""Create an association from a list of science exposures and a list of PSF reference exposures,
intended for calwebb_coron3 processing.
Parameters
----------
scifiles : list
List of science files
ref_targ_files : list
List of reference files
asnfile : str
The path to the association file.
"""
# Define the basic association of science files
asn = afl.asn_from_list(scifiles, rule=DMS_Level3_Base, product_name=prodname)
# Add reference target files to the association
nref = len(ref_targ_files)
for ii in range(0, nref):
asn['products'][0]['members'].append({'expname': ref_targ_files[ii], 'exptype': 'psf'})
# Write the association to a json file
_, serialized = asn.dump()
with open(asnfile, 'w') as outfile:
outfile.write(serialized)
Find and sort all of the input files, ensuring use of absolute paths. For the demo example there should be 2 science files and 9 PSF reference files.
# Science Files need the calints.fits files
sstring = os.path.join(image2_sci_r1_dir, 'jw*mirimage*calints.fits')
sstring2 = os.path.join(image2_sci_r2_dir, 'jw*mirimage*calints.fits')
r1_calfiles = sorted(glob.glob(sstring))
r2_calfiles = sorted(glob.glob(sstring2))
calfiles = r1_calfiles + r2_calfiles
# Check that these are the mask/filter to use
calfiles = select_mask_filter_files(calfiles, use_mask, use_filter)
# Reference target Files need the calints.fits files
sstring = os.path.join(image2_ref_targ_dir, 'jw*mirimage*calints.fits')
ref_targ_files = sorted(glob.glob(sstring))
# Check that these are the mask/filter to use
ref_targ_files = select_mask_filter_files(ref_targ_files, use_mask, use_filter)
print('Found ' + str(len(calfiles)) + ' science files to process')
print('Found ' + str(len(ref_targ_files)) + ' reference PSF files to process')
Found 2 science files to process
Found 9 reference PSF files to process
Make an association file that includes all of the Science and Reference files and run Coron3
if docoron3:
asnfile = os.path.join(coron3_dir, 'l3asn.json')
writel3asn(calfiles, ref_targ_files, asnfile, 'Level 3')
Coron3Pipeline.call(asnfile, steps=coron3dict, save_results=True, output_dir=coron3_dir)
else:
print('Skipping coron3 processing')
/usr/share/miniconda/lib/python3.13/site-packages/jwst/associations/association.py:234: UserWarning: Input association file contains path information; note that this can complicate usage and/or sharing of such files.
warnings.warn(err_str, UserWarning, stacklevel=1)
2025-12-18 19:56:24,377 - stpipe.step - INFO - PARS-RESAMPLESTEP parameters found: /home/runner/crds/references/jwst/miri/jwst_miri_pars-resamplestep_0001.asdf
2025-12-18 19:56:24,389 - stpipe.step - INFO - Coron3Pipeline instance created.
2025-12-18 19:56:24,390 - stpipe.step - INFO - StackRefsStep instance created.
2025-12-18 19:56:24,391 - stpipe.step - INFO - AlignRefsStep instance created.
2025-12-18 19:56:24,392 - stpipe.step - INFO - KlipStep instance created.
2025-12-18 19:56:24,393 - stpipe.step - INFO - OutlierDetectionStep instance created.
2025-12-18 19:56:24,395 - stpipe.step - INFO - ResampleStep instance created.
2025-12-18 19:56:24,600 - stpipe.step - INFO - Step Coron3Pipeline running with args ('./miri_coro_demo_data/stage3/l3asn.json',).
2025-12-18 19:56:24,608 - stpipe.step - INFO - Step Coron3Pipeline parameters are:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: ./miri_coro_demo_data/stage3
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: True
skip: False
suffix: i2d
search_output_file: True
input_dir: ''
steps:
stack_refs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
align_refs:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
median_box_length: 3
bad_bits: DO_NOT_USE
klip:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
truncate: 25
outlier_detection:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: False
input_dir: ''
weight_type: ivm
pixfrac: 1.0
kernel: square
fillval: NAN
maskpt: 0.7
snr: 5.0 4.0
scale: 1.2 0.7
backg: 0.0
kernel_size: 7 7
threshold_percent: 99.8
rolling_window_width: 25
ifu_second_check: False
save_intermediate_results: False
resample_data: True
good_bits: ~DO_NOT_USE
in_memory: True
resample:
pre_hooks: []
post_hooks: []
output_file: None
output_dir: None
output_ext: .fits
output_use_model: False
output_use_index: True
save_results: False
skip: False
suffix: None
search_output_file: True
input_dir: ''
pixfrac: 1.0
kernel: square
fillval: NAN
weight_type: exptime
output_shape: None
crpix: None
crval: None
rotation: None
pixel_scale_ratio: 1.0
pixel_scale: None
output_wcs: ''
single: False
blendheaders: True
in_memory: True
enable_ctx: True
enable_err: True
report_var: True
2025-12-18 19:56:24,610 - jwst.pipeline.calwebb_coron3 - INFO - Starting calwebb_coron3 ...
2025-12-18 19:56:25,859 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00001_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:25,861 - CRDS - INFO - Fetching /home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits 267.8 K bytes (1 / 1 files) (0 / 267.8 K bytes)
2025-12-18 19:56:26,264 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,291 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00002_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,293 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,320 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00003_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,322 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,349 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00004_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,352 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,379 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00005_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,381 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,408 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00006_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,411 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,438 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00007_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,440 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,468 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00008_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,470 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,498 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386007001_04101_00009_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,501 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,529 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386008001_04101_00001_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,531 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,558 - stpipe.pipeline - INFO - Prefetching reference files for dataset: 'jw01386009001_04101_00001_mirimage_calints.fits' reftypes = ['psfmask']
2025-12-18 19:56:26,560 - stpipe.pipeline - INFO - Prefetch for PSFMASK reference file is '/home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits'.
2025-12-18 19:56:26,894 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00001_mirimage_calints.fits>,).
2025-12-18 19:56:26,895 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:26,896 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:26,904 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:27,043 - jwst.outlier_detection.utils - INFO - 64 pixels marked as outliers
2025-12-18 19:56:27,187 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00001_mirimage_a3001_crfints.fits
2025-12-18 19:56:27,188 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:27,522 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00002_mirimage_calints.fits>,).
2025-12-18 19:56:27,523 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:27,524 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:27,531 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:27,672 - jwst.outlier_detection.utils - INFO - 64 pixels marked as outliers
2025-12-18 19:56:27,817 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00002_mirimage_a3001_crfints.fits
2025-12-18 19:56:27,818 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:28,156 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00003_mirimage_calints.fits>,).
2025-12-18 19:56:28,157 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:28,158 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:28,166 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:28,307 - jwst.outlier_detection.utils - INFO - 134 pixels marked as outliers
2025-12-18 19:56:28,452 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00003_mirimage_a3001_crfints.fits
2025-12-18 19:56:28,453 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:28,790 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00004_mirimage_calints.fits>,).
2025-12-18 19:56:28,791 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:28,792 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:28,800 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:28,940 - jwst.outlier_detection.utils - INFO - 138 pixels marked as outliers
2025-12-18 19:56:29,082 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00004_mirimage_a3001_crfints.fits
2025-12-18 19:56:29,083 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:29,415 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00005_mirimage_calints.fits>,).
2025-12-18 19:56:29,416 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:29,417 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:29,425 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:29,563 - jwst.outlier_detection.utils - INFO - 112 pixels marked as outliers
2025-12-18 19:56:29,705 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00005_mirimage_a3001_crfints.fits
2025-12-18 19:56:29,706 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:30,037 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00006_mirimage_calints.fits>,).
2025-12-18 19:56:30,038 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:30,038 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:30,047 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:30,184 - jwst.outlier_detection.utils - INFO - 171 pixels marked as outliers
2025-12-18 19:56:30,328 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00006_mirimage_a3001_crfints.fits
2025-12-18 19:56:30,329 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:30,663 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00007_mirimage_calints.fits>,).
2025-12-18 19:56:30,664 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:30,665 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:30,672 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:30,814 - jwst.outlier_detection.utils - INFO - 220 pixels marked as outliers
2025-12-18 19:56:30,959 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00007_mirimage_a3001_crfints.fits
2025-12-18 19:56:30,960 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:31,296 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00008_mirimage_calints.fits>,).
2025-12-18 19:56:31,297 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:31,298 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:31,306 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:31,444 - jwst.outlier_detection.utils - INFO - 234 pixels marked as outliers
2025-12-18 19:56:31,587 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00008_mirimage_a3001_crfints.fits
2025-12-18 19:56:31,588 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:31,922 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(19, 224, 288) from jw01386007001_04101_00009_mirimage_calints.fits>,).
2025-12-18 19:56:31,923 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:31,923 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:31,932 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:32,071 - jwst.outlier_detection.utils - INFO - 211 pixels marked as outliers
2025-12-18 19:56:32,215 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386007001_04101_00009_mirimage_a3001_crfints.fits
2025-12-18 19:56:32,216 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:32,452 - stpipe.step - INFO - Step stack_refs running with args (<jwst.datamodels.container.ModelContainer object at 0x7f2bf3959bd0>,).
2025-12-18 19:56:32,525 - jwst.coron.stack_refs - INFO - Adding psf member 1 to output stack
2025-12-18 19:56:32,537 - jwst.coron.stack_refs - INFO - Adding psf member 2 to output stack
2025-12-18 19:56:32,549 - jwst.coron.stack_refs - INFO - Adding psf member 3 to output stack
2025-12-18 19:56:32,561 - jwst.coron.stack_refs - INFO - Adding psf member 4 to output stack
2025-12-18 19:56:32,573 - jwst.coron.stack_refs - INFO - Adding psf member 5 to output stack
2025-12-18 19:56:32,585 - jwst.coron.stack_refs - INFO - Adding psf member 6 to output stack
2025-12-18 19:56:32,597 - jwst.coron.stack_refs - INFO - Adding psf member 7 to output stack
2025-12-18 19:56:32,609 - jwst.coron.stack_refs - INFO - Adding psf member 8 to output stack
2025-12-18 19:56:32,621 - jwst.coron.stack_refs - INFO - Adding psf member 9 to output stack
2025-12-18 19:56:32,681 - stpipe.step - INFO - Step stack_refs done
2025-12-18 19:56:32,844 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/Level 3_psfstack.fits
2025-12-18 19:56:33,203 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_calints.fits>,).
2025-12-18 19:56:33,204 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:56:33,205 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:56:33,214 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:56:33,479 - jwst.outlier_detection.utils - INFO - 33 pixels marked as outliers
2025-12-18 19:56:33,691 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386008001_04101_00001_mirimage_a3001_crfints.fits
2025-12-18 19:56:33,692 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:56:33,917 - stpipe.step - INFO - Step align_refs running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_a3001_crfints.fits>, <CubeModel(171, 224, 288) from Level 3_psfstack.fits>).
2025-12-18 19:56:33,966 - jwst.coron.align_refs_step - INFO - Using PSFMASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits
2025-12-18 19:57:22,838 - stpipe.step - INFO - Step align_refs done
2025-12-18 19:57:23,002 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386008001_04101_00001_mirimage_a3001_psfalign.fits
2025-12-18 19:57:23,222 - stpipe.step - INFO - Step klip running with args (<CubeModel(60, 224, 288) from jw01386008001_04101_00001_mirimage_a3001_crfints.fits>, <CubeModel(171, 224, 288) from jw01386008001_04101_00001_mirimage_a3001_psfalign.fits>).
2025-12-18 19:57:23,237 - jwst.coron.klip_step - INFO - KL transform truncation = 25
2025-12-18 19:57:36,053 - stpipe.step - INFO - Step klip done
2025-12-18 19:57:36,282 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386008001_04101_00001_mirimage_a3001_psfsub.fits
2025-12-18 19:57:40,260 - stpipe.step - INFO - Step outlier_detection running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_calints.fits>,).
2025-12-18 19:57:40,261 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection mode: coron
2025-12-18 19:57:40,262 - jwst.outlier_detection.outlier_detection_step - INFO - Outlier Detection asn_id: a3001
2025-12-18 19:57:40,271 - jwst.outlier_detection.utils - INFO - Computing median
2025-12-18 19:57:40,528 - jwst.outlier_detection.utils - INFO - 32 pixels marked as outliers
2025-12-18 19:57:40,745 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386009001_04101_00001_mirimage_a3001_crfints.fits
2025-12-18 19:57:40,746 - stpipe.step - INFO - Step outlier_detection done
2025-12-18 19:57:40,994 - stpipe.step - INFO - Step align_refs running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_a3001_crfints.fits>, <CubeModel(171, 224, 288) from Level 3_psfstack.fits>).
2025-12-18 19:57:41,012 - jwst.coron.align_refs_step - INFO - Using PSFMASK reference file /home/runner/crds/references/jwst/miri/jwst_miri_psfmask_0012.fits
2025-12-18 19:58:28,245 - stpipe.step - INFO - Step align_refs done
2025-12-18 19:58:28,402 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386009001_04101_00001_mirimage_a3001_psfalign.fits
2025-12-18 19:58:28,629 - stpipe.step - INFO - Step klip running with args (<CubeModel(60, 224, 288) from jw01386009001_04101_00001_mirimage_a3001_crfints.fits>, <CubeModel(171, 224, 288) from jw01386009001_04101_00001_mirimage_a3001_psfalign.fits>).
2025-12-18 19:58:28,643 - jwst.coron.klip_step - INFO - KL transform truncation = 25
2025-12-18 19:58:41,252 - stpipe.step - INFO - Step klip done
2025-12-18 19:58:41,487 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/jw01386009001_04101_00001_mirimage_a3001_psfsub.fits
2025-12-18 19:58:45,624 - stpipe.step - INFO - Step resample running with args (<jwst.datamodels.library.ModelLibrary object at 0x7f2bee4eb140>,).
2025-12-18 19:58:45,801 - jwst.resample.resample_utils - INFO - Pixel scale ratio (pscale_out/pscale_in): 1.0
2025-12-18 19:58:45,801 - jwst.resample.resample_utils - INFO - Computed output pixel scale: 0.11052169760859418 arcsec.
2025-12-18 19:58:45,953 - stcal.resample.resample - INFO - Output pixel scale: 0.11052169760859418 arcsec.
2025-12-18 19:58:45,954 - stcal.resample.resample - INFO - Driz parameter kernel: square
2025-12-18 19:58:45,955 - stcal.resample.resample - INFO - Driz parameter pixfrac: 1.0
2025-12-18 19:58:45,955 - stcal.resample.resample - INFO - Driz parameter fillval: NAN
2025-12-18 19:58:45,956 - stcal.resample.resample - INFO - Driz parameter weight_type: exptime
2025-12-18 19:58:45,957 - jwst.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:45,988 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:45,997 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,007 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,016 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,091 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,101 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,110 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,119 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,160 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,170 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,179 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,189 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,229 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,239 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,248 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,258 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,299 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,308 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,318 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,327 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,367 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,377 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,387 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,396 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,437 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,447 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,456 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,466 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,506 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,515 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,525 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,534 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,575 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,585 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,594 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,603 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,643 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,653 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,662 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,672 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,712 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,722 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,731 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,741 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,780 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,790 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,799 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,809 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,848 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,858 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,868 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,877 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,918 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,927 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,937 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,946 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:46,986 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:46,996 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,006 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,015 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,057 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,066 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,076 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,085 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,125 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,135 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,145 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,154 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,195 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,205 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,214 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,224 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,265 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,275 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,285 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,294 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,336 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,346 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,355 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,365 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,404 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,414 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,423 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,432 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,473 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,482 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,492 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,501 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,541 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,551 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,560 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,569 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,609 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,618 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,628 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,637 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,677 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,687 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,696 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,706 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,745 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,755 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,764 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,774 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,814 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,824 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,833 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,842 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,881 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,891 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,900 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,910 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,950 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:47,960 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,969 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:47,978 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,018 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,028 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,037 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,046 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,087 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,097 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,106 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,115 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,155 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,165 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,174 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,184 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,224 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,234 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,243 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,253 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,293 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,302 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,312 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,321 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,361 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,371 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,380 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,390 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,430 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,440 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,449 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,458 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,497 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,506 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,516 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,525 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,564 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,574 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,583 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,592 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,631 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,641 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,650 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,659 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,699 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,709 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,718 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,727 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,766 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,776 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,785 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,794 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,835 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,844 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,854 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,863 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,903 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,912 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,921 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,931 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,971 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:48,981 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:48,990 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,000 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,039 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,049 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,058 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,067 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,108 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,118 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,127 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,136 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,175 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,185 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,194 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,203 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,242 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,252 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,261 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,270 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,310 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,320 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,329 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,338 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,377 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,387 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,396 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,405 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,445 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,455 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,464 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,473 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,513 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,522 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,531 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,541 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,581 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,590 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,600 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,609 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,648 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,658 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,667 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,676 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,717 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,726 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,735 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,745 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,784 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,793 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,803 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,812 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,851 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,861 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,870 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,879 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,918 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,928 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,937 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,947 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:49,986 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:49,996 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,005 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,015 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,054 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,064 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,073 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,082 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,121 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,133 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,143 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,153 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,194 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,206 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,216 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,226 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,266 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,278 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,288 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,298 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,339 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,351 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,361 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,371 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,412 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,424 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,434 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,444 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,485 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,497 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,507 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,517 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,559 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,570 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,580 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,590 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,631 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,643 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,653 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,663 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,703 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,715 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,725 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,735 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,777 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,789 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,799 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,809 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,849 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,861 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,871 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,881 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,921 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:50,932 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,943 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,953 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:50,995 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,006 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,016 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,026 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,067 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,078 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,088 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,098 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,139 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,151 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,161 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,171 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,212 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,223 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,233 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,243 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,285 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,297 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,308 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,318 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,359 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,371 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,381 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,391 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,432 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,444 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,454 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,464 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,505 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,517 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,528 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,538 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,580 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,591 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,602 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,613 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,655 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,666 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,677 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,687 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,730 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,741 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,752 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,763 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,804 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,816 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,826 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,837 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,879 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,890 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,901 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,912 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,954 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:51,966 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,976 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:51,987 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,029 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,040 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,051 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,062 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,104 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,116 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,127 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,138 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,179 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,191 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,202 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,212 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,254 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,265 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,276 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,287 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,328 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,340 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,351 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,361 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,404 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,416 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,427 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,437 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,478 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,490 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,500 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,511 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,553 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,565 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,575 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,586 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,627 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,639 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,650 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,661 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,702 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,714 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,724 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,735 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,776 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,788 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,799 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,809 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,850 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,861 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,872 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,883 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,926 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:52,938 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,948 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:52,959 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,001 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,012 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,023 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,033 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,075 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,087 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,097 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,108 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,150 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,161 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,172 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,182 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,224 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,236 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,247 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,257 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,298 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,310 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,320 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,331 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,372 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,384 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,394 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,405 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,446 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,458 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,468 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,479 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,521 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,533 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,543 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,554 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,595 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,606 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,617 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,627 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,670 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,681 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,692 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,702 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,745 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,756 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,767 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,777 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,819 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,830 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,841 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,851 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,893 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,904 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,915 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,925 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,966 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:53,979 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:53,990 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,000 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,042 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,053 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,064 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,075 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,116 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,127 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,138 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,149 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,190 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,202 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,213 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,223 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,264 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,276 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,287 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,297 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,339 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,351 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,362 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,372 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,413 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,425 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,435 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,445 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,486 - stcal.resample.resample - INFO - Resampling science and variance data
2025-12-18 19:58:54,498 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,508 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,519 - stcal.resample.resample - INFO - Drizzling (224, 288) --> (269, 319)
2025-12-18 19:58:54,558 - stcal.alignment.util - INFO - Update S_REGION to POLYGON ICRS 201.141822823 -51.507192329 201.154429676 -51.509768023 201.159335067 -51.500462712 201.146730511 -51.497887543
2025-12-18 19:58:54,570 - stpipe.step - INFO - Step resample done
2025-12-18 19:58:54,954 - stpipe.step - INFO - Saved model in ./miri_coro_demo_data/stage3/Level 3_i2d.fits
2025-12-18 19:58:54,955 - jwst.pipeline.calwebb_coron3 - INFO - ...ending calwebb_coron3
2025-12-18 19:58:54,984 - stpipe.step - INFO - Step Coron3Pipeline done
2025-12-18 19:58:54,985 - jwst.stpipe.core - INFO - Results used jwst version: 1.20.2
Run calwebb_image3 using the call method.
# Print out the time benchmark
time1 = time.perf_counter()
print(f"Runtime so far: {time1 - time0:0.4f} seconds")
print(f"Runtime for coron3: {time1 - time_coron3} seconds")
Runtime so far: 1956.3368 seconds
Runtime for coron3: 150.83378939099998 seconds
8.-Examine the output#
Here we’ll plot the data to see what our source looks like.
# Stage 3 output files
# Individual exposures
sstring = os.path.join(coron3_dir, 'jw*psfsub.fits')
psfsubfiles = sorted(glob.glob(sstring))
npsfsub = len(psfsubfiles)
# Combined exposure
sstring = os.path.join(coron3_dir, '*i2d.fits')
i2dfiles = sorted(glob.glob(sstring))
if npsfsub == 1:
imgs = {'roll1': datamodels.open(psfsubfiles[0]).data.copy(),
'combo': datamodels.open(i2dfiles[0]).data.copy()}
else:
imgs = {'roll1': datamodels.open(psfsubfiles[0]).data.copy(),
'roll2': datamodels.open(psfsubfiles[1]).data.copy(),
'combo': datamodels.open(i2dfiles[0]).data.copy()}
fig, axes = plt.subplots(nrows=1, ncols=3, figsize=(16, 8))
vmin, vmax = np.nanquantile(np.concatenate(list([i.ravel() for i in imgs.values()])), [0.05, 0.95])
for i, roll in enumerate(imgs.keys()):
img = imgs[roll]
while img.ndim > 2:
img = np.nanmean(img, axis=0)
ax = axes[i]
ax.set_title(roll)
ax.imshow(img, vmin=vmin, vmax=vmax)
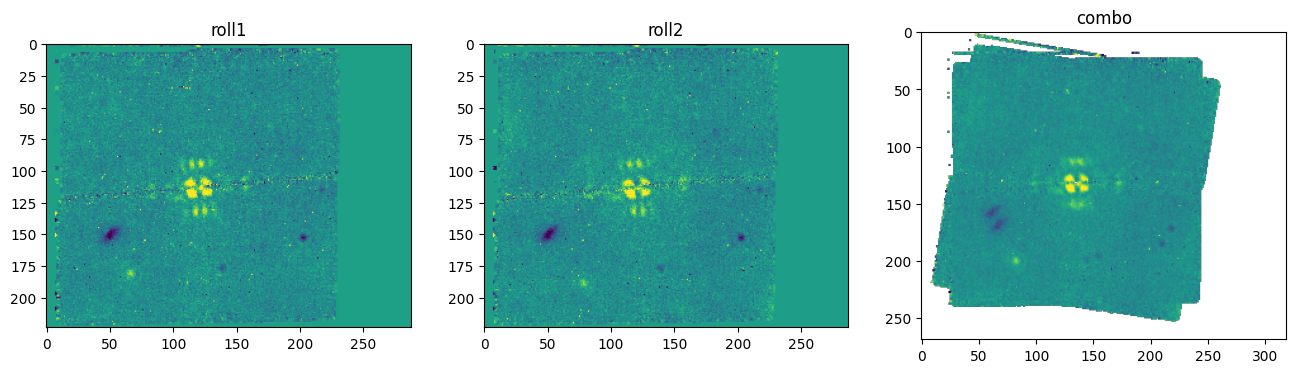
Overlay sky coordinates#
Overlay the RA and Dec grid over the combined rolls
with fits.open(i2dfiles[0]) as f:
wcs = WCS(f[1].header)
WARNING: FITSFixedWarning: 'datfix' made the change 'Set DATE-BEG to '2022-07-17T22:58:10.204' from MJD-BEG.
Set DATE-AVG to '2022-07-18T00:15:08.760' from MJD-AVG.
Set DATE-END to '2022-07-18T01:32:07.316' from MJD-END'. [astropy.wcs.wcs]
WARNING: FITSFixedWarning: 'obsfix' made the change 'Set OBSGEO-L to -69.981478 from OBSGEO-[XYZ].
Set OBSGEO-B to -37.692706 from OBSGEO-[XYZ].
Set OBSGEO-H to 1737840324.368 from OBSGEO-[XYZ]'. [astropy.wcs.wcs]
# The star coordinates at the time of observation are in the header
exp_file = uncal_sci_r1_files[0]
targ_ra = fits.getval(exp_file, 'TARG_RA', 0)
targ_dec = fits.getval(exp_file, 'TARG_DEC', 0)
starcoord = SkyCoord(targ_ra, targ_dec, unit='deg', frame='icrs')
fig, ax = plt.subplots(1, 1, subplot_kw={'projection': wcs})
vmin, vmax = np.nanquantile(imgs['combo'], [0.01, 0.99])
ax.imshow(imgs['combo'], vmin=vmin, vmax=vmax)
ax.scatter(*wcs.world_to_pixel(starcoord),
marker='x', s=100, c='w')
ax.grid(True)


